Stuttering associated with a pathogenic variant in the chaperone protein cyclophilin 40
- PMID: 37977818
- PMCID: PMC10689913
- DOI: 10.1093/brain/awad314
Stuttering associated with a pathogenic variant in the chaperone protein cyclophilin 40
Abstract
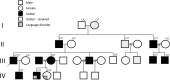
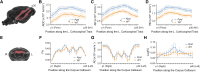
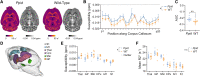
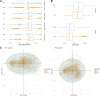
Stuttering is a common speech disorder that interrupts speech fluency and tends to cluster in families. Typically, stuttering is characterized by speech sounds, words or syllables which may be repeated or prolonged and speech that may be further interrupted by hesitations or 'blocks'. Rare variants in a small number of genes encoding lysosomal pathway proteins have been linked to stuttering. We studied a large four-generation family in which persistent stuttering was inherited in an autosomal dominant manner with disruption of the cortico-basal-ganglia-thalamo-cortical network found on imaging. Exome sequencing of three affected family members revealed the PPID c.808C>T (p.Pro270Ser) variant that segregated with stuttering in the family. We generated a Ppid p.Pro270Ser knock-in mouse model and performed ex vivo imaging to assess for brain changes. Diffusion-weighted MRI in the mouse revealed significant microstructural changes in the left corticospinal tract, as previously implicated in stuttering. Quantitative susceptibility mapping also detected changes in cortico-striatal-thalamo-cortical loop tissue composition, consistent with findings in affected family members. This is the first report to implicate a chaperone protein in the pathogenesis of stuttering. The humanized Ppid murine model recapitulates network findings observed in affected family members.
Keywords: PPID gene; brain MRI; chaperone; cyclophilin-40; stuttering.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
I.E.S. has served on scientific advisory boards for UCB, Eisai, GlaxoSmithKline, BioMarin, Nutricia, Rogcon, Chiesi, Encoded Therapeutics, Knopp Biosciences and Xenon Pharmaceuticals; has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, Chiesi, Liva Nova and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin and Eisai; has served as an investigator for Zogenix, Zynerba, Ultragenyx, GW Pharma, UCB, Eisai, Xenon Pharmaceuticals, Anavex Life Sciences, Ovid Therapeutics, Epigenyx, Encoded Therapeutics and Marinus; and has consulted for Zynerba Pharmaceuticals, Atheneum Partners, Ovid Therapeutics, Care Beyond Diagnosis, Epilepsy Consortium and UCB; and is a Non-Executive Director of Bellberry Ltd. She may accrue future revenue on pending patent WO61/010176 (filed: 2008): Therapeutic Compound; has a patent for SCN1A testing held by Bionomics Inc. and licensed to various diagnostic companies; has a patent molecular diagnostic/theranostic target for benign familial infantile epilepsy (BFIE) [PRRT2] 2011904493 & 2012900190 and PCT/AU2012/001321 (TECH ID:2012-009). A.P.V. works for and owns stock in Redenlab Inc., an audio analysis company. The remaining authors report no competing interests.
Figures
Comment in
-
New insights into the genetics of stuttering.Brain. 2023 Dec 1;146(12):4788-4790. doi: 10.1093/brain/awad369. Brain. 2023. PMID: 37987612 No abstract available.
References
-
- Craig A, Hancock K, Tran Y, Craig M, Peters K. Epidemiology of stuttering in the community across the entire life span. J Speech Lang Hear Res. 2002;45:1097–1105. - PubMed
-
- Riley GD. A stuttering severity instrument for children and adults. J Speech Hear Disord. 1972;37:314–322. - PubMed
-
- Boyce JO, Jackson VE, van Reyk O, et al. Self-reported impact of developmental stuttering across the lifespan. Dev Med Child Neurol. 2022;64:1297–1306. - PubMed
-
- Kang C, Drayna D. Genetics of speech and language disorders. Annu Rev Genomics Hum Genet. 2011;12:145–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

