Functional Dissection of Ipsilateral and Contralateral Neural Activity Propagation Using Voltage-Sensitive Dye Imaging in Mouse Prefrontal Cortex
- PMID: 37977827
- PMCID: PMC10706255
- DOI: 10.1523/ENEURO.0161-23.2023
Functional Dissection of Ipsilateral and Contralateral Neural Activity Propagation Using Voltage-Sensitive Dye Imaging in Mouse Prefrontal Cortex
Abstract
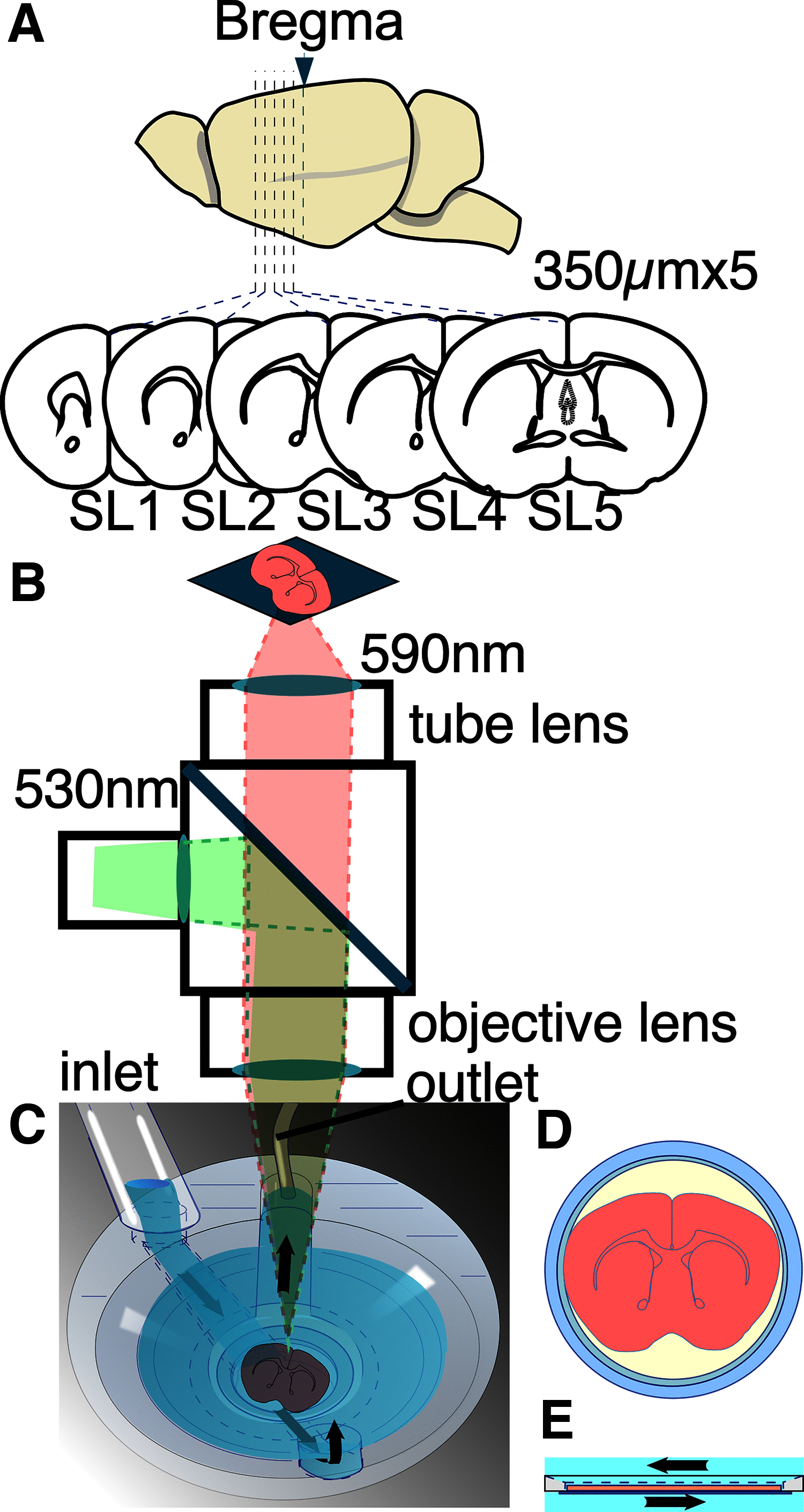
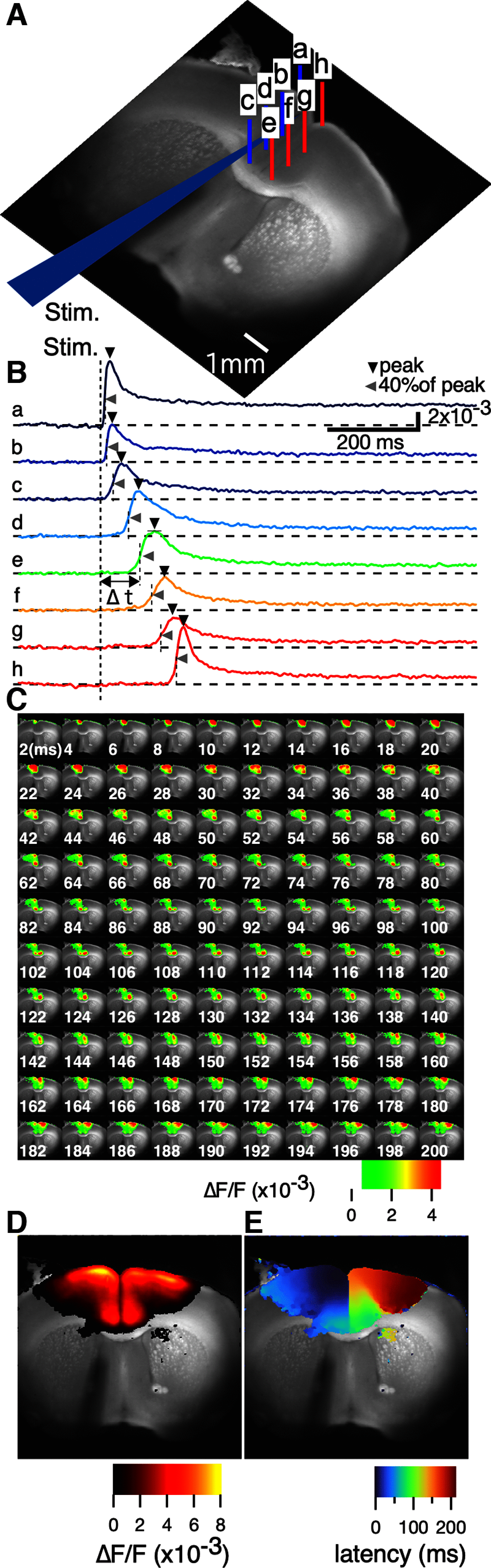
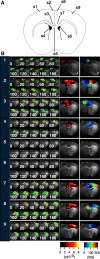
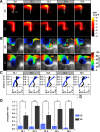
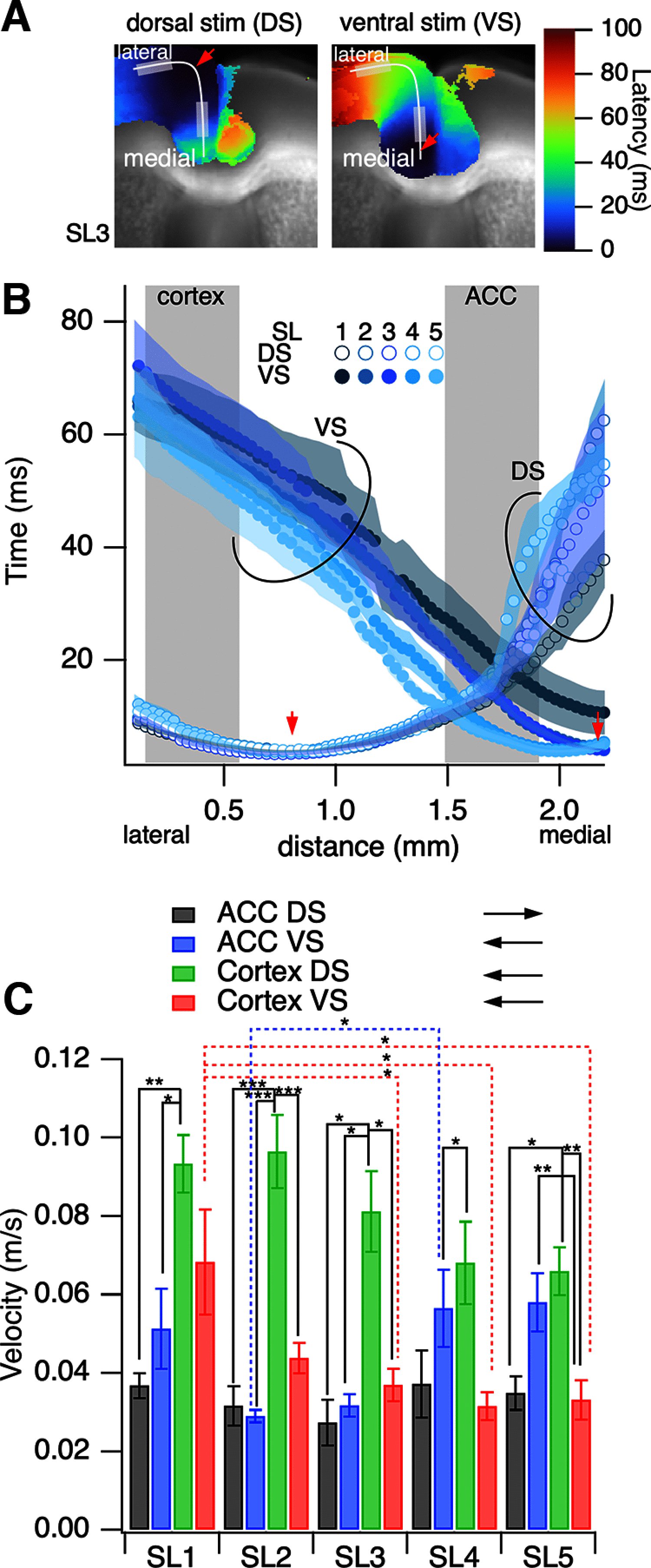
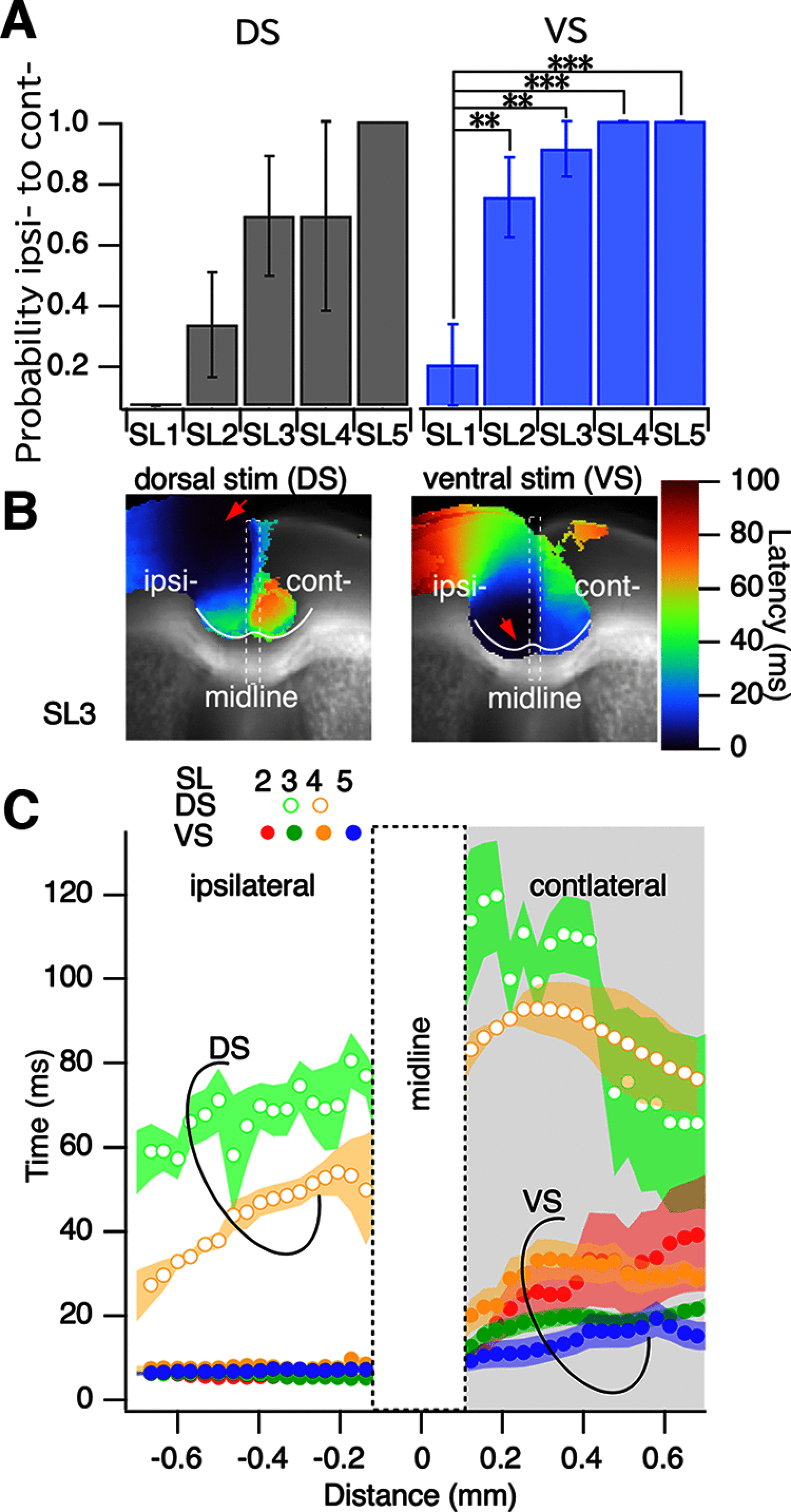
Prefrontal cortex (PFC) intrahemispheric activity and the interhemispheric connection have a significant impact on neuropsychiatric disorder pathology. This study aimed to generate a functional map of FC intrahemispheric and interhemispheric connections. Functional dissection of mouse PFCs was performed using the voltage-sensitive dye (VSD) imaging method with high speed (1 ms/frame), high resolution (256 × 256 pixels), and a large field of view (∼10 mm). Acute serial 350 μm slices were prepared from the bregma covering the PFC and numbered 1-5 based on their distance from the bregma (i.e., 1.70, 1.34, 0.98, 0.62, and 0.26 mm) with reference to the Mouse Brain Atlas (Paxinos and Franklin, 2008). The neural response to electrical stimulation was measured at nine sites and then averaged, and a functional map of the propagation patterns was created. Intracortical propagation was observed in slices 3-5, encompassing the anterior cingulate cortex (ACC) and corpus callosum (CC). The activity reached area 33 of the ACC. Direct white matter stimulation activated area 33 in both hemispheres. Similar findings were obtained via DiI staining of the CC. Imaging analysis revealed directional biases in neural signals traveling within the ACC, whereby the signal transmission speed and probability varied based on the signal direction. Specifically, the spread of neural signals from cg2 to cg1 was stronger than that from cingulate cortex area 1(cg1) to cingulate cortex area 2(cg2), which has implications for interhemispheric functional connections. These findings highlight the importance of understanding the PFC functional anatomy in evaluating neuromodulators like serotonin and dopamine, as well as other factors related to neuropsychiatric diseases.
Keywords: anterior cingulate cortex; corpus callosum; medial prefrontal cortex; voltage-sensitive dye.
Copyright © 2023 Gusain et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Optical Assay of the Functional Impact of Cuprizone-Induced Demyelination and Remyelination on Interhemispheric Neural Communication in the Anterior Cingulate Cortex via the Corpus Callosum.eNeuro. 2025 Jan 6;12(1):ENEURO.0511-24.2024. doi: 10.1523/ENEURO.0511-24.2024. Print 2025 Jan. eNeuro. 2025. PMID: 39746809 Free PMC article.
-
Intra- and Interhemispheric Propagation of Electrophysiological Synchronous Activity and Its Modulation by Serotonin in the Cingulate Cortex of Juvenile Mice.PLoS One. 2016 Mar 1;11(3):e0150092. doi: 10.1371/journal.pone.0150092. eCollection 2016. PLoS One. 2016. PMID: 26930051 Free PMC article.
-
Voltage-sensitive-dye imaging of microstimulation-evoked neural activity through intracortical horizontal and callosal connections in cat visual cortex.J Neural Eng. 2009 Dec;6(6):066002. doi: 10.1088/1741-2560/6/6/066002. Epub 2009 Oct 1. J Neural Eng. 2009. PMID: 19794238
-
What, If Anything, Is Rodent Prefrontal Cortex?eNeuro. 2018 Oct 25;5(5):ENEURO.0315-18.2018. doi: 10.1523/ENEURO.0315-18.2018. eCollection 2018 Sep-Oct. eNeuro. 2018. PMID: 30406193 Free PMC article. Review.
-
Short-term synaptic plasticity in the nociceptive thalamic-anterior cingulate pathway.Mol Pain. 2009 Sep 4;5:51. doi: 10.1186/1744-8069-5-51. Mol Pain. 2009. PMID: 19732417 Free PMC article. Review.
Cited by
-
Berberine alleviates chronic pain-induced anxiety-like behaviors by inhibiting the activation of VLT-projecting cACC (Cg2) neurons.Commun Biol. 2024 Dec 19;7(1):1651. doi: 10.1038/s42003-024-07372-2. Commun Biol. 2024. PMID: 39702401 Free PMC article.
-
Optical Assay of the Functional Impact of Cuprizone-Induced Demyelination and Remyelination on Interhemispheric Neural Communication in the Anterior Cingulate Cortex via the Corpus Callosum.eNeuro. 2025 Jan 6;12(1):ENEURO.0511-24.2024. doi: 10.1523/ENEURO.0511-24.2024. Print 2025 Jan. eNeuro. 2025. PMID: 39746809 Free PMC article.
-
Action of GABAB receptor on local network oscillation in somatosensory cortex of oral part: focusing on NMDA receptor.J Physiol Sci. 2024 Mar 12;74(1):16. doi: 10.1186/s12576-024-00911-w. J Physiol Sci. 2024. PMID: 38475711 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
