An open-label study evaluating the safety and efficacy of budesonide in patients with IgA nephropathy at high risk of progression
- PMID: 37978255
- PMCID: PMC10656480
- DOI: 10.1038/s41598-023-47393-1
An open-label study evaluating the safety and efficacy of budesonide in patients with IgA nephropathy at high risk of progression
Abstract
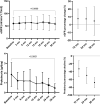
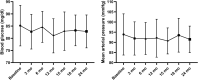
We sought to evaluate the efficacy and safety of budesonide (Budenofalk) in the treatment of patients with IgA Nephropathy. We conducted a prospective, interventional, open-label, single-arm, non-randomized study that enrolled 32 patients with IgAN at high risk of progression (BUDIGAN study, ISRCTN47722295, date of registration 14/02/2020). Patients were treated with Budesonide at a dose of 9 mg/day for 12 months, subsequently tapered to 3 mg/day for another 12 months. The primary endpoints were change of eGFR and proteinuria at 12, 24 and 36 months. The study cohort had a mean eGFR and 24-h proteinuria of 59 ± 24 ml/min/1.73m2 and 1.89 ± 1.5 g/day, respectively. Treatment with budesonide determined a reduction in proteinuria at 12-, 24- and 36-months by -32.9% (95% CI - 53.6 to - 12.2), - 49.7% (95% CI - 70.1 to - 29.4) and - 68.1% (95% CI - 80.6 to - 55.7). Budesonide determined an eGFR preservation corresponding to a 12-, 24- and 36-months change of + 7.68% (95% CI - 4.7 to 20.1), + 7.42% (95% CI - 7.23 to 22.1) and + 4.74% (95%CI - 13.5 to 23), respectively. The overall eGFR change/year was + 0.83 ml/min/y (95% CI - 0.54 to 4.46). Budesonide was well-tolerated, and treatment emergent adverse events were mostly mild in severity and reversible. Budesonide was effective in the treatment of patients with IgAN at high-risk of progression in terms of reducing proteinuria and preserving renal function over 36 months of therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

