The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial
- PMID: 37978284
- PMCID: PMC10803270
- DOI: 10.1038/s41591-023-02668-y
The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial
Erratum in
-
Author Correction: The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial.Nat Med. 2024 May;30(5):1500. doi: 10.1038/s41591-024-02910-1. Nat Med. 2024. PMID: 38467878 Free PMC article. No abstract available.
-
Author Correction: The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial.Nat Med. 2025 Jul;31(7):2454. doi: 10.1038/s41591-025-03709-4. Nat Med. 2025. PMID: 40240838 Free PMC article. No abstract available.
Abstract
BRAF genomic alterations are the most common oncogenic drivers in pediatric low-grade glioma (pLGG). Arm 1 (n = 77) of the ongoing phase 2 FIREFLY-1 (PNOC026) trial investigated the efficacy of the oral, selective, central nervous system-penetrant, type II RAF inhibitor tovorafenib (420 mg m-2 once weekly; 600 mg maximum) in patients with BRAF-altered, relapsed/refractory pLGG. Arm 2 (n = 60) is an extension cohort, which provided treatment access for patients with RAF-altered pLGG after arm 1 closure. Based on independent review, according to Response Assessment in Neuro-Oncology High-Grade Glioma (RANO-HGG) criteria, the overall response rate (ORR) of 67% met the arm 1 prespecified primary endpoint; median duration of response (DOR) was 16.6 months; and median time to response (TTR) was 3.0 months (secondary endpoints). Other select arm 1 secondary endpoints included ORR, DOR and TTR as assessed by Response Assessment in Pediatric Neuro-Oncology Low-Grade Glioma (RAPNO) criteria and safety (assessed in all treated patients and the primary endpoint for arm 2, n = 137). The ORR according to RAPNO criteria (including minor responses) was 51%; median DOR was 13.8 months; and median TTR was 5.3 months. The most common treatment-related adverse events (TRAEs) were hair color changes (76%), elevated creatine phosphokinase (56%) and anemia (49%). Grade ≥3 TRAEs occurred in 42% of patients. Nine (7%) patients had TRAEs leading to discontinuation of tovorafenib. These data indicate that tovorafenib could be an effective therapy for BRAF-altered, relapsed/refractory pLGG. ClinicalTrials.gov registration: NCT04775485 .
© 2023. The Author(s).
Conflict of interest statement
L.B.K. has received consulting fees from Blueprint Medicine as DSMB Chair and has contracted institutional research with Novartis, Regeneron Pharmaceuticals, Day One Biopharmaceuticals, Spring Works Therapeutics, Bristol Myers Squibb and SonALAsense. L.B.K. also owns stock in Onconova Therapeutics. J.R.H. has received honoraria for consultation from Bayer, Alexion Pharma and Boxer Capital. P.H.D. is on an advisory board with Alexion and is part of the Alexion ICI Sprinkle Study. S.P. is on advisory boards with Bayer, Alexion and Esai and has received research support from Novartis, Bayer and Roche. D.S.Z. has received consulting/advisory board fees from Bayer, AstraZeneca, Accendatech, Novartis, Day One Biopharmaceuticals, FivePhusion, Amgen, Alexion and Norgine and has received research support from Accendatech. O.W. is on advisory boards with Novartis, Janssen, Roche, Bristol Myers Squibb and AstraZeneca and has received research grants from Day One Biopharmaceuticals, Biomed Valley Discovery, Bristol Myers Squibb, Syndax and PreComb. H.J.K. is on advisory boards with Novartis, Jazz Pharmaceuticals, Takeda, Cartexell and GPCR. D.H. is on advisory boards with Alexion/AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Day One Biopharmaceuticals, Janssen, Novartis and Roche and has received research grants from Alexion/AstraZeneca and Roche. V.L. is on an advisory board with Alexion. C.K. is a study chair of an investigator-sponsored trial for which Day One Biopharmaceuticals provides drug and research support; she also has research relationships with other industry partners. D.S. is on an advisory board with Alexion. L.M., X.Z., A.W., D.D., P.M., I.C. and S.C.B. are employees of Day One Biopharmaceuticals and have received Day One Biopharmaceuticals stock and stock options. K.N. has received advisory board or consulting fees from Y-mAbs, EUSA Pharma, Bayer and Eli Lilly. All remaining authors declare no competing interests.
Figures
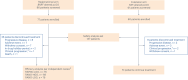
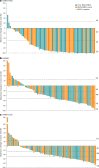
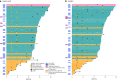
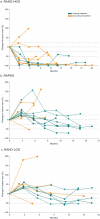

References
-
- Gnekow, A. K. et al. SIOP-E-BTG and GPOH guidelines for diagnosis and treatment of children and adolescents with low grade glioma. Klin. Padiatr.231, 107–135 (2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

