A role of TRIM59 in pulmonary hypertension: modulating the protein ubiquitylation modification
- PMID: 37978515
- PMCID: PMC10655329
- DOI: 10.1186/s12967-023-04712-4
A role of TRIM59 in pulmonary hypertension: modulating the protein ubiquitylation modification
Abstract
Background: Pulmonary hypertension (PH), an infrequent disease, is characterized by excessive pulmonary vascular remodeling and proliferation of pulmonary artery smooth muscle cells (PASMCs). However, its underlying molecular mechanisms remain unclear. Uncovering its molecular mechanisms will be beneficial to the treatment of PH.
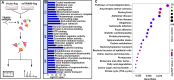
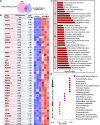
Methods: Differently expressed genes (DEGs) in the lung tissues of PH patients were analyzed with a GEO dataset GSE113439. From these DEGs, we focused on TRIM59 which was highly expressed in PH patients. Subsequently, the expression of TRIM59 in the pulmonary arteries of PH patients, lung tissues of PH rat model and PASMCs cultured in a hypoxic condition was verified by quantitative real-time PCR (qPCR), western blot and immunohistochemistry. Furthermore, the role of TRIM59 in PAMSC proliferation and pathological changes in PH rats was assessed via gain-of-function and loss-of-function experiments. In addition, the transcriptional regulation of YAP1/TEAD4 on TRIM59 was confirmed by qPCR, western blot, luciferase reporter assay, ChIP and DNA pull-down. In order to uncover the underlying mechanisms of TRIM59, a protein ubiquitomics and a CoIP- HPLC-MS/MS were companied to identify the direct targets of TRIM59.
Results: TRIM59 was highly expressed in the pulmonary arteries of PH patients and lung tissues of PH rats. Over-expression of TRIM59 accelerated the proliferation of PASMCs, while TRIM59 silencing resulted in the opposite results. Moreover, TRIM59 silencing mitigated the injuries in heart and lung and attenuated pulmonary vascular remodeling during PH. In addition, its transcription was positively regulated by YAP1/TEAD4. Then we further explored the underlying mechanisms of TRIM59 and found that TRIM59 overexpression resulted in an altered ubiquitylation of proteins. Accompanied with the results of CoIP- HPLC-MS/MS, 34 proteins were identified as the direct targets of TRIM59.
Conclusion: TRIM59 was highly expressed in PH patients and promoted the proliferation of PASMCs and pulmonary vascular remodeling, thus contributing to the pathogenesis of PH. It is indicated that TRIM59 may become a potential target for PH treatment.
Keywords: Pulmonary artery smooth muscle cells; Pulmonary hypertension; TRIM59; Ubiquitylation modification; YAP1/TEAD4.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Humbert M, Guignabert C, Bonnet S, Dorfmuller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. - DOI - PMC - PubMed
-
- Zhang L, Wang Y, Wu G, Rao L, Wei Y, Yue H, Yuan T, Yang P, Xiong F, Zhang S, et al. Blockade of JAK2 protects mice against hypoxia-induced pulmonary arterial hypertension by repressing pulmonary arterial smooth muscle cell proliferation. Cell Prolif. 2020;53:e12742. doi: 10.1111/cpr.12742. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

