Improvements in quality of life over 2 years with cladribine tablets in people with relapsing multiple sclerosis: The CLARIFY-MS study
- PMID: 37978852
- PMCID: PMC10687821
- DOI: 10.1177/13524585231205962
Improvements in quality of life over 2 years with cladribine tablets in people with relapsing multiple sclerosis: The CLARIFY-MS study
Abstract
Background: Multiple sclerosis (MS) negatively affects health-related quality of life (HRQoL).
Objective: To evaluate HRQoL in people with highly active relapsing MS treated with cladribine tablets (CladT; 3.5 mg/kg cumulative dose over 2 years) in CLARIFY-MS.
Methods: Changes in the MS quality of life (MSQoL)-54 scores were analysed using a repeated mixed-effects linear model. Subgroup analyses were performed for participants who were pretreatment-naïve and those pretreated with disease-modifying therapies (DMTs) before initiating CladT. Safety and tolerability of CladT were also assessed.
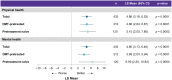
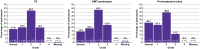
Results: MSQoL-54 physical (mean change = 4.86; 95% confidence interval (CI) = 3.18, 6.53) and mental health (4.80; 95% CI = 3.13, 6.46) composite scores (primary endpoints) showed significant improvement at Month 24 versus Baseline (p < 0.0001). Changes in the MSQoL-54 scores were consistent across the pretreatment-naïve and DMT-pretreated subgroups. No new severe or opportunistic infections occurred. Most post-baseline lymphopenia events were Grade 1-2 in severity. Transient Grade-3 lymphopenia was observed in 19.7% (95/482) of participants. Grade-4 lymphopenia was not observed.
Conclusions: CladT treatment significantly improved the mean MSQoL-54 physical and mental health composite scores over 2 years. CladT efficacy in HRQoL, relapse rates and Expanded Disability Status Scale scores demonstrates its multidimensional effects in MS treatment.
Trial registration: ClinicalTrials.gov NCT03369665.
Keywords: CLARIFY-MS; Cladribine tablets; disease-modifying therapies; multiple sclerosis; quality of life.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: B.B. has received consultancy fees, speaker fees, research grants (non-personal) or honoraria from Biogen, Celgene (Bristol Myers Squibb (BMS)), Merck, Novartis, Roche and Sanofi. A.S. has served on advisory boards for Merck, Novartis and Sanofi, and has been invited to speak on behalf of Almirall, Biogen, EXCEMED, Merck and Teva. J.L.-S. has accepted travel compensation from Biogen, Merck and Novartis; her institution receives the honoraria for talks and advisory board commitment, and research grants from Biogen, Celgene (BMS), Merck, Novartis, Roche, Sanofi and Teva. F.P. has received research grants from Janssen, Merck and Sanofi, and fees for serving as a member of the data monitoring committee (DMC) in clinical trials with Lundbeck, Chugai and Roche, and preparation of witness statement for Novartis. D.L. has participated in speaker bureau for Almirall, Bayer, Biogen, BMS, Merck, Novartis, Roche, Sanofi and Teva; has received consultancy fees from Bayer, Biogen, BMS, Merck, Novartis and Teva; and has received research grants from Bayer, Biogen, Merck and Novartis. R.H. has received institutional research grants and fees for lectures and advisory boards from Biogen, Merck and Sanofi. K.S. has received honoraria for speaking, consulting and serving for advisory boards for Biogen, Celgene (BMS), Merck, Novartis, Roche and TG Therapeutics. F.Pa. has served on scientific Advisory Boards for Almirall, Bayer, Biogen, Celgene (BMS), Merck, Novartis, Roche, Sanofi and Teva; he also received speaker honoraria from the same companies and non-personal research grants for his department from Biogen, Merck, Novartis and Sanofi. L.B. has received honoraria, travel expenses, speaker fees and advisory fees from Almirall, Bayer, Celgene (BMS), Biogen, Merck, Novartis, Roche, Sanofi and Teva. E.M.M. has received honoraria for participating as primary investigator in clinical trials from Actelion (Janssen/J&J), Merck, Novartis and Teva. N.A., A.Sm., A.N. and B.K. are employees of Merck Healthcare KGaA, Darmstadt, Germany. X.M. has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with AbbVie, Actelion, Alexion, Biogen, BMS/Celgene, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Genzyme, Hoffmann-La Roche, Immunic, Janssen Pharmaceuticals, MedDay, Merck, Mylan, NervGen, Novartis, Sandoz, Sanofi-Genzyme, Teva Pharmaceutical, TG Therapeutics, EXCEMED, MSIF and NMSS. E.K.H. has received honoraria/research support from Actelion (Janssen/J&J), Biogen, Celgene (BMS), Merck, Novartis, Roche, Sanofi and Teva; has served on advisory boards for Actelion (Janssen/J&J), Biogen, Celgene (BMS), Merck, Novartis, Roche and Sanofi; has been supported by the Czech Ministry of Education – project Cooperatio LF1, research area Neuroscience and the project National Institute for Neurological Research (Programme EXCELES, ID project No LX22NPO5107) – funded by the European Union-Next Generation EU.
Figures


Comment in
-
Improved quality of life with MS treatment.Nat Rev Neurol. 2024 Jan;20(1):1. doi: 10.1038/s41582-023-00913-z. Nat Rev Neurol. 2024. PMID: 38049642 No abstract available.
References
-
- Diaz C, Zarco LA, Rivera DM. Highly active multiple sclerosis: An update. Mult Scler Relat Disord 2019; 30: 215–224. - PubMed
-
- Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. - PubMed
-
- He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: A retrospective observational cohort study. Lancet Neurol 2020; 19(4): 307–316. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

