The Crohn's Disease Exclusion Diet: A Comprehensive Review of Evidence, Implementation Strategies, Practical Guidance, and Future Directions
- PMID: 37978895
- PMCID: PMC11446999
- DOI: 10.1093/ibd/izad255
The Crohn's Disease Exclusion Diet: A Comprehensive Review of Evidence, Implementation Strategies, Practical Guidance, and Future Directions
Abstract
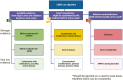
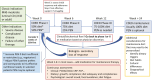
Dietary therapy is increasingly recognized for the management of Crohn's disease (CD) over recent years, including the use of exclusive enteral nutrition (EEN) as first-line therapy for pediatric CD according to current guidelines. The Crohn's disease exclusion diet (CDED) is a whole-food diet designed to reduce exposure to dietary components that are potentially pro-inflammatory, mediated by negative effects on the gut microbiota, immune response, and the intestinal barrier. The CDED has emerged as a valid alternative to EEN with cumulative evidence, including randomized controlled trials, supporting use for induction of remission and possibly maintenance in children and adults. We gathered a group of multidisciplinary experts, including pediatric and adult gastroenterologists, inflammatory bowel diseases (IBD) expert dietitians, and a psychologist to discuss the evidence, identify gaps, and provide insights into improving the use of CDED based on a comprehensive review of CDED literature and professional experience. This article reviews the management of CDED in both children and adults, long-term aspects of CDED, indications and contraindications, selecting the best candidates, identifying challenges with CDED, globalization, the role of the multidisciplinary team, especially of dietitian, and future directions. We concluded that CDED is an established dietary therapy that could serve as an alternative to EEN in many pediatric and adult cases, especially with mild to moderate disease. In severe disease, complicated phenotypes, or with extraintestinal involvement, CDED should be considered on a case-by-case basis, according to physician and dietitians' discretion. More studies are warranted to assess the efficacy of CDED in different scenarios.
Keywords: Crohn’s disease; exclusion diets; gut microbiome; nutritional therapy.
Plain language summary
The Crohn’s disease exclusion diet (CDED) has emerged as an alternative to exclusive enteral nutrition for the treatment of pediatric Crohn’s disease. In this review, we summarize data on efficacy and challenges and identify research priorities, clinical gaps, and opportunities.
© 2023 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
R.S.B. received funds for consulting or speaker from Nestlé Health Science, Takeda, Megapharm and Janssen; served on advisory board for Evinature and Nestlé Health Science.
C.W. received funds for consulting, speaker and research from Nestlé Health Sciences.
C.S.S.: Wolfson medical center IP for Nestle Health Science, and speaking fees from Nestle, Takeda.
L.A. received funds for consulting or speaking from Nestle Health Sciences, Abbott Nutrition.
P.L. received conference and/or consulting fees from Takeda, Nestlè Health Science, Janssen, Sandoz, Firma Menarini, and Nutricia.
V.M.N-L. received funds for consulting or speaking from AbbVie, Mead-Johnson Nutrition, Nestle Health Sciences, Adacyte and Ordesa.
F.J.M.C. received honoraria for talks and advisory from Abbvie, Nestle, MSD, Adacyte, Janssen.
H.Y. has received speaking and/ or consulting fees from Pfizer, Abbvie, Lilly, Takeda, Janssen, BMS, and grant support from Pfizer.
N.M. has received speaking and/ or consulting fees from Pfizer, Abbvie, Lilly, Takeda, Janssen, Ferring, BiomX, BMS, Nestle, Trobix and grant support from Takeda, Janssen, Abbott, Abbvie, Pfizer, BMS, Corundum Innovation Ltd, Nestle and from the Helmsely Charitable Trust.
J.V.L. was supported by funding from the Amsterdam UMC; Wetenschappelijke Advies Raad of Stichting Steun Emma (Emma Children’s Hospital); the Department of Pediatrics, Amsterdam University Medical Centers; a Pro-KIIDS Clinical Research Network Award (#585718) and a Life Sciences and Health Public Private Partnership of Health Holland and Nestle Health Science. J.V.L. received research funds from Nestle Health Science. J.V.L. received funds for consulting and speaking from Nestle Health Science, Pfizer, and Nutricia and for participation in advisory boards from Nestle Health Science and Pfizer.
E.W. received funds for consulting or speaking from AbbVie, BioJamp, Janssen, Mead-Johnson Nutrition, Nestle Health Sciences, and Pfizer.
I.O. has no conflicts to declare.
Figures



References
-
- Fitzpatrick JA, Melton SL, Yao CK, Gibson PR, Halmos EP.. Dietary management of adults with IBD - the emerging role of dietary therapy. Nat Rev Gastroenterol Hepatol. 2022;19(10):652-669. - PubMed
-
- Levine A, Rhodes JM, Lindsay JO, et al. Dietary guidance from the International Organization for the Study of inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18(6):1381-1392. - PubMed
-
- Sohouli MH, Fatahi S, Farahmand F, et al. Meta-analysis: efficacy of exclusive enteral nutrition as induction therapy on disease activity index, inflammation and growth factors in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2022;56(3):384-395. - PubMed
-
- van Rheenen PF, Aloi M, Assa A, et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. 2021;15(2):171-194. - PubMed
-
- Lawley M, Wu JW, Navas-Lopez VM, et al. Global variation in use of enteral nutrition for pediatric Crohn’s disease. J Pediatr Gastroenterol Nutr. 2018;67(2):e22-e29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

