Time toxicity associated with early phase clinical trial participation
- PMID: 37979324
- PMCID: PMC10774969
- DOI: 10.1016/j.esmoop.2023.102046
Time toxicity associated with early phase clinical trial participation
Abstract
Background: Early phase cancer clinical trials (EPCTs) involve experimental drugs being used for the first time in humans. These studies are designed for dose determination and safety, and represent the most time intensive of all clinical trials for both clinicians and patients. We sought to quantify the amount of patient time consumed through EPCT participation.
Patients and methods: A retrospective audit of patients treated in the EPCT unit at Liverpool Hospital, Sydney was carried out from 2013 to 2023. We defined 'time toxicity' (TT) as a composite measure where time-toxic days were considered days with any health care system contact, including clinic visits, infusions, procedures or blood work.
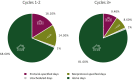
Results: A total of 219 patients across 36 EPCTs were included. The median age was 65 years (range 31-81 years). Patients spent a median of 29% (range 4%-100%) of their days in direct contact with the health care system during their study. Protocol-specified visits accounted for the greatest contribution to total TT in 101 (46%) patients. In 7% (n = 16) of patients, unscheduled visits due to either adverse events or cancer-related symptoms accounted for the greatest TT. TT reduced as patients completed additional cycles of treatment. Patients who completed >10 cycles spent 14% of their days interacting with health care systems compared with 35% for those who completed ≤2 cycles. No statistically significant difference in TT was noted between dose-expansion and dose-escalation studies or trials focusing on immune-oncology versus targeted therapy.
Conclusions: Our study is the first to report TT in EPCTs with an extended follow-up. Clinicians should be aware of TT when discussing risks and benefits. TT also may not be the appropriate term when describing the time patients invest during EPCTs. Toxicity implies a negative impact, but for many patients, trial participation would be seen as positive. There should be efforts to streamline health care visits to limit TT in EPCTs.
Keywords: early phase clinical trials; phase 1; risk versus benefit; shared decision making; time toxicity.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure The authors have declared no conflicts of interest.
Figures
Similar articles
-
Comparison of Prognostic Scores in Early Phase Clinical Trials: A 10-year Single Centre Australian Experience.Anticancer Res. 2024 May;44(5):2095-2102. doi: 10.21873/anticanres.17014. Anticancer Res. 2024. PMID: 38677731
-
A Phase I/II Clinical Trial to evaluate the efficacy of baricitinib to prevent respiratory insufficiency progression in onco-hematological patients affected with COVID19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Feb 5;22(1):116. doi: 10.1186/s13063-021-05072-4. Trials. 2021. PMID: 33546739 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care.Health Technol Assess. 2001;5(21):1-75. doi: 10.3310/hta5210. Health Technol Assess. 2001. PMID: 11532238 Review.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
Cited by
-
Social and Legal Needs in Patients and Families With Cancer: Interaction With Patient-Level Financial Toxicity.JCO Oncol Pract. 2025 Jan;21(1):41-51. doi: 10.1200/OP.24.00305. Epub 2025 Jan 10. JCO Oncol Pract. 2025. PMID: 39793548 Review.
-
Improving Access to Patient-Focused, Decentralized Clinical Trials Requires Streamlined Regulatory Requirements: An ASCO Research Statement.J Clin Oncol. 2024 Nov 20;42(33):3986-3995. doi: 10.1200/JCO.24.00961. Epub 2024 Jul 30. J Clin Oncol. 2024. PMID: 39079075
-
"The Cancer is My Life": patient and caregiver perceptions of the time toxicity of palliative systemic cancer treatments for advanced gastrointestinal cancers.Support Care Cancer. 2025 Jun 10;33(7):564. doi: 10.1007/s00520-025-09621-4. Support Care Cancer. 2025. PMID: 40493254 Free PMC article.
-
Time toxicity in cancer care: A concept analysis using Walker and Avant's method.Asia Pac J Oncol Nurs. 2024 Oct 23;11(12):100610. doi: 10.1016/j.apjon.2024.100610. eCollection 2024 Dec. Asia Pac J Oncol Nurs. 2024. PMID: 39641009 Free PMC article. Review.
-
Patient, Caregiver, and Clinician Perspectives on the Time Burdens of Cancer Care.JAMA Netw Open. 2024 Nov 4;7(11):e2447649. doi: 10.1001/jamanetworkopen.2024.47649. JAMA Netw Open. 2024. PMID: 39602118 Free PMC article.
References
-
- Thomas E.E., Kelly J.T., Taylor M.L., et al. Telehealth adoption in cancer clinical trials: an Australian perspective. Asia Pac J Clin Oncol. 2022;19:549–558. - PubMed
-
- Gupta A., Eisenhauer E.A., Booth C.M. The time toxicity of cancer treatment. J Clin Oncol. 2022;40(15):1611–1615. - PubMed
-
- Araujo D., Greystoke A., Bates S., et al. Oncology phase I trial design and conduct: time for a change - MDICT guidelines 2022. Ann Oncol. 2023;34(1):48–60. - PubMed
-
- Durbin S., Lundquist D., Healy M., et al. Time toxicity in early phase clinical trials (EP-CTs) J Clin Oncol. 2022;40(suppl 28):236.
-
- Simmons M., Hetrick S., Jorm A. Shared decision-making: benefits, barriers and current opportunities for application. Australas Psychiatry. 2010;18(5):394–397. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical