Efficacy and safety of avacopan in patients with ANCA-associated vasculitis receiving rituximab in a randomised trial
- PMID: 37979959
- PMCID: PMC10850685
- DOI: 10.1136/ard-2023-224816
Efficacy and safety of avacopan in patients with ANCA-associated vasculitis receiving rituximab in a randomised trial
Abstract
Objectives: To evaluate the efficacy and safety of avacopan in the subgroup of patients with antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis receiving background induction therapy with rituximab in the phase 3 ADVOCATE trial.
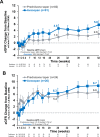
Methods: Key efficacy outcomes were remission at week 26 and sustained remission at week 52. Additional outcomes included the Glucocorticoid Toxicity Index, estimated glomerular filtration rate, urinary albumin to creatinine ratio, health-related quality of life and safety.
Results: Of the 330 patients who received study medication, 214 (64.8%) received rituximab (once weekly for 4 weeks), with a mean age of 59.8 years; 163 (76.2%) had renal vasculitis and 125 (58.4%) were newly diagnosed. Remission at week 26 and sustained remission at week 52 were achieved by 83/107 (77.6%) and 76/107 (71.0%) patients in the avacopan group and 81/107 (75.7%) and 60/107 (56.1%) in the prednisone taper group, respectively. The relapse rate, recovery of renal function, speed of reduction in albuminuria and glucocorticoid toxicity favoured the avacopan group. Serious adverse events occurred in 34.6% and 39.3% of patients in the avacopan and prednisone taper groups, respectively.
Conclusions: These data suggest that in patients with ANCA-associated vasculitis receiving rituximab, efficacy of treatment with avacopan compared with a prednisone taper was similar at week 26 and greater at week 52, with a favourable safety profile. In addition, avacopan was associated with improved renal outcomes and lower glucocorticoid toxicity. These results demonstrate the efficacy and safety of avacopan in patients receiving background induction therapy with rituximab.
Trial registration number: NCT02994927.
Keywords: Glucocorticoids; Granulomatosis with polyangiitis; Rituximab; Systemic vasculitis.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DG reports consultant fees from Amgen, ChemoCentryx (a wholly owned subsidiary of Amgen), Aurinia, Otsuka, Calliditas Therapeutics, and GlaxoSmithKline (GSK). AD reports consultant fees from Amgen, ChemoCentryx (a wholly owned subsidiary of Amgen), Novartis, Sanofi, AbbVie and GSK, grants from the Rheumatology Research Foundation, advisory board participation for Sanofi and GSK, honoraria and travel support for lectures from GSK, and a leadership role at the Vasculitis Foundation and Chicago Rheumatism Society. HY reports stocks from and was an employee of ChemoCentryx (a wholly owned subsidiary of Amgen) and Amgen. JS reports consultant fees from ChemoCentryx (a wholly owned subsidiary of Amgen). CS reports royalties from UpToDate and consultant fees from Amgen, Novartis, CSL Vifor, Lilly, Boehringer Ingelheim, Pfizer and Jannsen. DJ reports consultant fees from AstraZeneca, Boehringer Ingelheim, ChemoCentryx (a wholly owned subsidiary of Amgen), GSK, Novartis, Roche and CSL Vifor, and grant or research support from GSK and Roche. PM reports royalties from UpToDate, consultant fees from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, ChemoCentryx (a wholly owned subsidiary of Amgen), Eicos, Electra, Forbius, Genentech/Roche, GSK, InflaRx, Neutrolis, Novartis, Sanofi and Takeda, support for travel to a scientific meeting from ChemoCentryx (a wholly owned subsidiary of Amgen), stock options from Kyverna and Sparrow, and grants to his institution from the National Institutes of Health, Food and Drug Administration, Vasculitis Foundation, American College of Rheumatology and EULAR.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

