Comparison of relative change with effect size metrics in Alzheimer's disease clinical trials
- PMID: 37979967
- PMCID: PMC11299058
- DOI: 10.1136/jnnp-2023-331941
Comparison of relative change with effect size metrics in Alzheimer's disease clinical trials
Abstract
Background: Per cent slowing of decline is frequently used as a metric of outcome in Alzheimer's disease (AD) clinical trials, but it may be misleading. Our objective was to determine whether per cent slowing of decline or Cohen's d is the more valid and informative measure of efficacy.
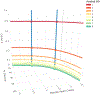
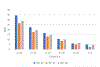
Methods: Outcome measures of interest were per cent slowing of decline; Cohen's d effect size and number-needed-to-treat (NNT). Data from a graphic were used to model the inter-relationships among Cohen's d, placebo decline in raw score units and per cent slowing of decline with active treatment. NNTs were computed based on different magnitudes of d. Last, we tabulated recent AD anti-amyloid clinical trials that reported per cent slowing and for which we computed their respective d's and NNTs.
Results: We demonstrated that d and per cent slowing were potentially independent. While per cent slowing of decline was dependent on placebo decline and did not include variance in its computation, d was dependent on both group mean difference and pooled SD. We next showed that d was a critical determinant of NNT, such that NNT was uniformly smaller when d was larger. In recent AD associated trials including those focused on anti-amyloid biologics, d's were below 0.23 and thus considered small, while per cent slowing was in the 22-29% range and NNTs ranged from 14 to 18.
Conclusions: Standardised effect size is a more meaningful outcome than per cent slowing of decline because it determines group overlap, which can directly influence NNT computations, and yield information on the likelihood of minimum clinically important differences. In AD, greater use of effect sizes, NNTs, rather than relative per cent slowing, will improve the ability to interpret clinical trial results and evaluate the clinical meaningfulness of statistically significant results.
Keywords: ALZHEIMER'S DISEASE; COGNITION; DEMENTIA; STATISTICS.
© Author(s) (or their employer(s)) 2024. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LSS reports personal fees from AC Immune, Alpha-cognition, Athira, Corium, Cortexyme, BioVie, Eli Lilly, GW Research, Lundbeck, Merck, Neurim, Novo-Nordisk, Otsuka, Roche/Genentech, Cognition Therapeutics, Takeda; grants from Biohaven, Biogen, Eisai, Eli Lilly and Novartis. DPD reports research support from the National Institute on Aging, Alzheimer’s Association, is a scientific adviser to Acadia, TauRx, Corium, Genentech, and is a member of the Data and Safety Monitoring Board of BioXcel. TG and SL have no conflicts of interest to disclose.
Figures
Comment in
-
How effective is effective enough?J Neurol Neurosurg Psychiatry. 2023 Dec 14;95(1):1. doi: 10.1136/jnnp-2023-332311. J Neurol Neurosurg Psychiatry. 2023. PMID: 37989567 No abstract available.
References
-
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed: Lawrence Erlbaum Associates; 1988.
-
- Furukawa TA. From effect size into number needed to treat. Lancet. 1999;353(9165):1680. - PubMed
-
- van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in Early Alzheimer's Disease. N Engl J Med. 2023;388(1):9–21. - PubMed
-
- Magnusson K. Interpreting Cohen’s d Effect Size: An interactive visualization R Psychologist 2022. [updated September 19, 2022. Available from: https://rpsychologist.com/cohend/.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
