Inhibition of UFM1 expression suppresses cancer progression and is linked to the dismal prognosis and immune infiltration in oral squamous cell carcinoma
- PMID: 37980168
- PMCID: PMC10713408
- DOI: 10.18632/aging.205219
Inhibition of UFM1 expression suppresses cancer progression and is linked to the dismal prognosis and immune infiltration in oral squamous cell carcinoma
Abstract
Background: Ubiquitin fold modifier 1 (UFM1) overexpression is associated with cancer cell proliferation, migration and invasion. However, the roles and pathways of UFM1 in oral squamous cell carcinoma (OSCC) has remained undefined.
Methods: The expression of UFM1 and the relationship between UFM1 expression and prognosis were investigated using data of OSCC patients from The Cancer Genome Atlas (TCGA) database. The UFM1 co-expressed genes, and the association between the UFM1 expression and immune cells and ubiquitination were explored. The effects of UFM1 expression on the growth and migration of OSCC cells were investigated by siRNA interference, Cell Counting Kit-8 (CCK-8), Transwell, Western blotting, and wound healing experiments.
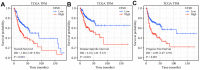
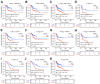
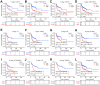
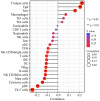
Results: UFM1 was highly expressed in OSCC. UFM1 overexpression was associated with short overall survival, disease-specific survival, and progression-free interval, and was an adverse factor for prognosis in OSCC. UFM1-related nomograms were significantly associated with poor prognosis in OSCC patients. Decreased UFM1 expression could inhibit the proliferation, migration, and invasion of OSCC cells. UFM1 was associated with the immune cells (such as the Th17 cells, T helper cells, and cytotoxic cells) and ubiquitination.
Conclusion: Elevated UFM1 expression was associated with poor prognosis, ubiquitination and immune infiltration in OSCC, and inhibition of UFM1 expression delayed OSCC progression, showing that UFM1 could be a biomarker for prognosis and treating OSCC patients.
Keywords: OSCC; UFM1; biomarkers; immune cells; ubiquitination.
Conflict of interest statement
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

