Iron derived from NCOA4-mediated ferritinophagy causes cellular senescence via the cGAS-STING pathway
- PMID: 37980349
- PMCID: PMC10657394
- DOI: 10.1038/s41420-023-01712-7
Iron derived from NCOA4-mediated ferritinophagy causes cellular senescence via the cGAS-STING pathway
Abstract
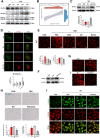
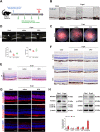
Cellular senescence is a hallmark of aging and has been linked to age-related diseases. Age-related macular degeneration (AMD), the most common aging-related retinal disease, is prospectively associated with retinal pigment epithelial (RPE) senescence. However, the mechanism of RPE cell senescence remains unknown. In this study, tert-butyl hydroperoxide (TBH)-induced ARPE-19 cells and D-galactose-treated C57 mice were used to examine the cause of elevated iron in RPE cell senescence. Ferric ammonium citrate (FAC)-treated ARPE-19 cells and C57 mice were used to elucidated the mechanism of iron overload-induced RPE cell senescence. Molecular biology techniques for the assessment of iron metabolism, cellular senescence, autophagy, and mitochondrial function in vivo and in vitro. We found that iron level was increased during the senescence process. Ferritin, a major iron storage protein, is negatively correlated with intracellular iron levels and cell senescence. NCOA4, a cargo receptor for ferritinophagy, mediates degradation of ferritin and contributes to iron accumulation. Besides, we found that iron overload leads to mitochondrial dysfunction. As a result, mitochondrial DNA (mtDNA) is released from damaged mitochondria to cytoplasm. Cytoplasm mtDNA activates the cGAS-STING pathway and promotes inflammatory senescence-associated secretory phenotype (SASP) and cell senescence. Meanwhile, iron chelator Deferoxamine (DFO) significantly rescues RPE senescence and retinopathy induced by FAC or D-gal in mice. Taken together, these findings imply that iron derived from NCOA4-mediated ferritinophagy causes cellular senescence via the cGAS-STING pathway. Inhibiting iron accumulation may represent a promising therapeutic approach for age-related diseases such as AMD.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

