Adaptive traits of cysts of the snow alga Sanguina nivaloides unveiled by 3D subcellular imaging
- PMID: 37980360
- PMCID: PMC10657455
- DOI: 10.1038/s41467-023-43030-7
Adaptive traits of cysts of the snow alga Sanguina nivaloides unveiled by 3D subcellular imaging
Abstract
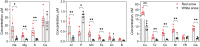
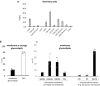
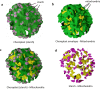
Sanguina nivaloides is the main alga forming red snowfields in high mountains and Polar Regions. It is non-cultivable. Analysis of environmental samples by X-ray tomography, focused-ion-beam scanning-electron-microscopy, physicochemical and physiological characterization reveal adaptive traits accounting for algal capacity to reside in snow. Cysts populate liquid water at the periphery of ice, are photosynthetically active, can survive for months, and are sensitive to freezing. They harbor a wrinkled plasma membrane expanding the interface with environment. Ionomic analysis supports a cell efflux of K+, and assimilation of phosphorus. Glycerolipidomic analysis confirms a phosphate limitation. The chloroplast contains thylakoids oriented in all directions, fixes carbon in a central pyrenoid and produces starch in peripheral protuberances. Analysis of cells kept in the dark shows that starch is a short-term carbon storage. The biogenesis of cytosolic droplets shows that they are loaded with triacylglycerol and carotenoids for long-term carbon storage and protection against oxidative stress.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Remias D. Cell structure and physiology of alpine snow and ice algae. In: Plants in Alpine Regions (ed Lütz C.). Springer (2012).
-
- Aristotle. History of animals / Περί ζῴων ἱστορίαι. (c. 347-322 BC).
-
- Plinius C. Natural history / Naturalis historia. (c. 77).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

