Phase 1b study of enzalutamide plus CC-115, a dual mTORC1/2 and DNA-PK inhibitor, in men with metastatic castration-resistant prostate cancer (mCRPC)
- PMID: 37980367
- PMCID: PMC10781677
- DOI: 10.1038/s41416-023-02487-5
Phase 1b study of enzalutamide plus CC-115, a dual mTORC1/2 and DNA-PK inhibitor, in men with metastatic castration-resistant prostate cancer (mCRPC)
Abstract
Background: CC-115, a dual mTORC1/2 and DNA-PK inhibitor, has promising antitumour activity when combined with androgen receptor (AR) inhibition in pre-clinical models.
Methods: Phase 1b multicentre trial evaluating enzalutamide with escalating doses of CC-115 in AR inhibitor-naive mCRPC patients (n = 41). Primary endpoints were safety and RP2D. Secondary endpoints included PSA response, time-to-PSA progression, and radiographic progression.
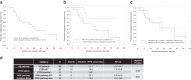
Results: Common adverse effects included rash (31.7% Grades 1-2 (Gr); 31.7% Gr 3), pruritis (43.9% Gr 1-2), diarrhoea (37% Gr 1-2), and hypertension (17% Gr 1-2; 9.8% Gr 3). CC-115 RP2D was 5 mg twice a day. In 40 evaluable patients, 80% achieved ≥50% reduction in PSA (PSA50), and 58% achieved ≥90% reduction in PSA (PSA90) by 12 weeks. Median time-to-PSA progression was 14.7 months and median rPFS was 22.1 months. Stratification by PI3K alterations demonstrated a non-statistically significant trend towards improved PSA50 response (PSA50 of 94% vs. 67%, p = 0.08). Exploratory pre-clinical analysis suggested CC-115 inhibited mTOR pathway strongly, but may be insufficient to inhibit DNA-PK at RP2D.
Conclusions: The combination of enzalutamide and CC-115 was well tolerated. A non-statistically significant trend towards improved PSA response was observed in patients harbouring PI3K pathway alterations, suggesting potential predictive biomarkers of response to a PI3K/AKT/mTOR pathway inhibitor.
Clinical trial registration: ClinicalTrials.gov identifier: NCT02833883.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
JLZ is currently a full-time employee at AstraZeneca, which is not involved in the funding and conduct of the trial. The authors declare no competing interests.
Figures



References
-
- American Cancer Society. Cancer Statistics Center. 2022. http://cancerstatisticscenter.cancer.org.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

