PET/CT based cross-modal deep learning signature to predict occult nodal metastasis in lung cancer
- PMID: 37980411
- PMCID: PMC10657428
- DOI: 10.1038/s41467-023-42811-4
PET/CT based cross-modal deep learning signature to predict occult nodal metastasis in lung cancer
Abstract
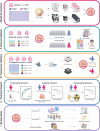
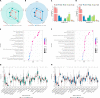
Occult nodal metastasis (ONM) plays a significant role in comprehensive treatments of non-small cell lung cancer (NSCLC). This study aims to develop a deep learning signature based on positron emission tomography/computed tomography to predict ONM of clinical stage N0 NSCLC. An internal cohort (n = 1911) is included to construct the deep learning nodal metastasis signature (DLNMS). Subsequently, an external cohort (n = 355) and a prospective cohort (n = 999) are utilized to fully validate the predictive performances of the DLNMS. Here, we show areas under the receiver operating characteristic curve of the DLNMS for occult N1 prediction are 0.958, 0.879 and 0.914 in the validation set, external cohort and prospective cohort, respectively, and for occult N2 prediction are 0.942, 0.875 and 0.919, respectively, which are significantly better than the single-modal deep learning models, clinical model and physicians. This study demonstrates that the DLNMS harbors the potential to predict ONM of clinical stage N0 NSCLC.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Lung cancer with PET/CT-defined occult nodal metastasis yields favourable prognosis and benefits from adjuvant therapy: a multicentre study.Eur J Nucl Med Mol Imaging. 2022 Jun;49(7):2414-2424. doi: 10.1007/s00259-022-05690-3. Epub 2022 Jan 20. Eur J Nucl Med Mol Imaging. 2022. PMID: 35048154
-
Limitations of PET/CT in the Detection of Occult N1 Metastasis in Clinical Stage I(T1-2aN0) Non-Small Cell Lung Cancer for Staging Prior to Stereotactic Body Radiotherapy.Technol Cancer Res Treat. 2017 Feb;16(1):15-21. doi: 10.1177/1533034615624045. Epub 2016 Jun 23. Technol Cancer Res Treat. 2017. PMID: 26792491 Free PMC article.
-
Clinical Predictors of Nodal Metastases in Peripherally Clinical T1a N0 Non-Small Cell Lung Cancer.Ann Thorac Surg. 2017 Oct;104(4):1153-1158. doi: 10.1016/j.athoracsur.2017.02.074. Epub 2017 May 24. Ann Thorac Surg. 2017. PMID: 28551047
-
Use of Magnetic Resonance Imaging for N-Staging in Patients with Non-Small Cell Lung Cancer. A Systematic Review.Arch Bronconeumol (Engl Ed). 2019 Jan;55(1):9-16. doi: 10.1016/j.arbres.2018.03.007. Epub 2018 May 24. Arch Bronconeumol (Engl Ed). 2019. PMID: 29803524
-
Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography.Eur J Cardiothorac Surg. 2009 Sep;36(3):440-5. doi: 10.1016/j.ejcts.2009.04.003. Epub 2009 May 22. Eur J Cardiothorac Surg. 2009. PMID: 19464906 Review.
Cited by
-
CT-based radiomics-deep learning model predicts occult lymph node metastasis in early-stage lung adenocarcinoma patients: A multicenter study.Chin J Cancer Res. 2025 Jan 30;37(1):12-27. doi: 10.21147/j.issn.1000-9604.2025.01.02. Chin J Cancer Res. 2025. PMID: 40078558 Free PMC article.
-
Recent Advances in PET and Radioligand Therapy for Lung Cancer: FDG and FAP.Cancers (Basel). 2025 Aug 1;17(15):2549. doi: 10.3390/cancers17152549. Cancers (Basel). 2025. PMID: 40805245 Free PMC article. Review.
-
Multiregional dynamic contrast-enhanced MRI-based integrated system for predicting pathological complete response of axillary lymph node to neoadjuvant chemotherapy in breast cancer: multicentre study.EBioMedicine. 2024 Sep;107:105311. doi: 10.1016/j.ebiom.2024.105311. Epub 2024 Aug 26. EBioMedicine. 2024. PMID: 39191174 Free PMC article.
-
Hypermetabolic pulmonary lesions detection and diagnosis based on PET/CT imaging and deep learning models.Eur J Nucl Med Mol Imaging. 2025 Aug;52(10):3792-3806. doi: 10.1007/s00259-025-07215-0. Epub 2025 Apr 4. Eur J Nucl Med Mol Imaging. 2025. PMID: 40183951 Free PMC article.
-
A novel nomogram based on PET/CT to predict lymph nodal metastasis for lung adenocarcinoma with normal size lymph node.J Cardiothorac Surg. 2025 Apr 16;20(1):202. doi: 10.1186/s13019-025-03443-5. J Cardiothorac Surg. 2025. PMID: 40241103 Free PMC article.
References
-
- Darling GE, et al. Positron emission tomography-computed tomography compared with invasive mediastinal staging in non-small cell lung cancer: results of mediastinal staging in the early lung positron emission tomography trial. J. Thorac. Oncol. 2011;6:1367–1372. doi: 10.1097/JTO.0b013e318220c912. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

