Development and optimisation of in vitro sonodynamic therapy for glioblastoma
- PMID: 37980454
- PMCID: PMC10657375
- DOI: 10.1038/s41598-023-47562-2
Development and optimisation of in vitro sonodynamic therapy for glioblastoma
Abstract
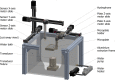
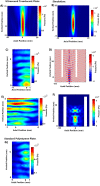
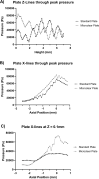
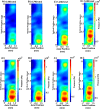
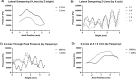
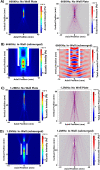
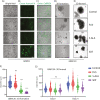
Sonodynamic therapy (SDT) is currently on critical path for glioblastoma therapeutics. SDT is a non-invasive approach utilising focused ultrasound to activate photosensitisers like 5-ALA to impede tumour growth. Unfortunately, the molecular mechanisms underlying the therapeutic functions of SDT remain enigmatic. This is primarily due to the lack of intricately optimised instrumentation capable of modulating SDT delivery to glioma cells in vitro. Consequently, very little information is available on the effects of SDT on glioma stem cells which are key drivers of gliomagenesis and recurrence. To address this, the current study has developed and validated an automated in vitro SDT system to allow the application and mapping of focused ultrasound fields under varied exposure conditions and setup configurations. The study optimizes ultrasound frequency, intensity, plate base material, thermal effect, and the integration of live cells. Indeed, in the presence of 5-ALA, focused ultrasound induces apoptotic cell death in primary patient-derived glioma cells with concurrent upregulation of intracellular reactive oxygen species. Intriguingly, primary glioma stem neurospheres also exhibit remarkably reduced 3D growth upon SDT exposure. Taken together, the study reports an in vitro system for SDT applications on tissue culture-based disease models to potentially benchmark the novel approach to the current standard-of-care.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

