Altered fecal bile acid composition in active ulcerative colitis
- PMID: 37980492
- PMCID: PMC10656844
- DOI: 10.1186/s12944-023-01971-4
Altered fecal bile acid composition in active ulcerative colitis
Abstract
Background: Disturbed bile acid homeostasis associated with a rise of primary and a decline of secondary bile acids is a consistent finding in inflammatory bowel diseases (IBDs). Whether fecal bile acids may emerge as biomarkers for IBD diagnosis and disease severity is less clear. Our study aimed to identify associations of 18 fecal bile acid species with IBD entity and disease activity.
Methods: Stool samples of 62 IBD patients and 17 controls were collected. Eighteen fecal bile acid species were quantified by LC-MS/MS using stable isotope dilution. Lipid levels normalized to a dry weight of the fecal homogenates and ratios of single bile acid species to total bile acid levels were used for calculations.
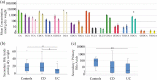
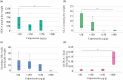
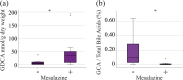
Results: IBD patients exhibited altered primary and secondary bile acid ratios in stool, with notable distinctions between ulcerative colitis (UC) compared to Crohn's disease (CD) and healthy controls. Fecal calprotectin was negatively correlated with glycolithocholic acid (GLCA) and hyodeoxycholic acid (HDCA) in UC. These bile acids were reduced in stool of UC patients with fecal calprotectin levels > 500 µg/g compared to UC patients with low calprotectin levels. Moreover, negative associations of six secondary bile acids with C-reactive protein (CRP) existed in UC. In CD patients, fecal bile acids did not correlate with CRP or fecal calprotectin. Diarrhoea is common in IBD, and UC patients with diarrhoea had reduced deoxycholic acid (DCA), glycine conjugated DCA (GDCA) and lithocholic acid in stool in contrast to patients with normal stool consistency. Fecal bile acid levels were not associated with diarrhoea in CD patients. UC patients treated with mesalazine had increased levels of fecal GDCA whereas no such changes were observed in CD patients. Bile acid levels of CD and UC patients treated with biologicals or corticosteroids did not change. Relative levels of GHDCA (specificity: 79%, sensitivity: 67%) and glycochenodeoxycholic acid (specificity: 74%, sensitivity: 63%) were the most specific to distinguish UC from CD.
Conclusion: Disrupted fecal bile acid homeostasis is associated with disease severity and disease symptoms in UC but not in CD, potentially aiding in distinguishing IBD subtypes and classifying the pathophysiology of diarrhoea in UC.
Keywords: Bile acids; Cholic acid; Crohn´s Disease; Deoxycholic acid; Fecal calprotectin; Ulcerative Colitis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Gnewuch C, Liebisch G, Langmann T, Dieplinger B, Mueller T, Haltmayer M, Dieplinger H, Zahn A, Stremmel W, Rogler G, Schmitz G. Serum bile acid profiling reflects enterohepatic detoxification state and intestinal barrier function in inflammatory bowel disease. World J Gastroenterol. 2009;15:3134–3141. doi: 10.3748/wjg.15.3134. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

