Cu (II)-catalyzed: synthesis of imidazole derivatives and evaluating their larvicidal, antimicrobial activities with DFT and molecular docking studies
- PMID: 37980500
- PMCID: PMC10657005
- DOI: 10.1186/s13065-023-01067-1
Cu (II)-catalyzed: synthesis of imidazole derivatives and evaluating their larvicidal, antimicrobial activities with DFT and molecular docking studies
Abstract
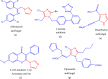
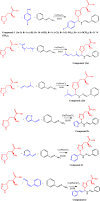
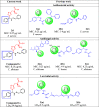
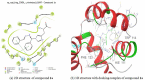
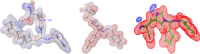
This paper deals with the evaluation of novel imidazole molecules for their antimicrobial and larvicidal activities. A series of imidazole derivatives 1(a-f) and 2(a-e) were prepared by the Mannich base technique using a Cu(II) catalyst. The Cu(phen)Cl2 catalyst was found to be more effective than other methods. FTIR, elemental analyses, mass spectrometry, 1H NMR, and 13C NMR spectroscopy were performed to elucidate the structures of the synthesised compounds. Antimicrobial and larvicidal activities were investigated for all compounds. The antibacterial activity of compounds (2d) and (2a) were highly active in S.aureus (MIC: 0.25 μg/mL) and K.pneumoniae (MIC: 0.25 μg/mL) compared to ciprofloxacin. Compound (1c) was significantly more effective than clotrimazole in C.albicans (MIC: 0.25 μg/mL). Molecular docking studies of compound 2d showed a higher binding affinity for the 1BDD protein (- 3.4 kcal/mol) than ciprofloxacin (- 4.4 kcal/mol). Compound 1c had a higher binding affinity (- 6.0 kcal/mol) than clotrimazole (- 3.1 kcal/mol) with greater frontier molecular orbital energy and reactivity properties of compound 1c (∆E gap = 0.13 eV). The activity of compound 1a (LD50: 34.9 μg/mL) was more effective in the Culex quinquefasciatus than permethrin (LD50: 35.4 μg/mL) and its molecular docking binding affinity for 3OGN protein (- 6.1 kcal/mol). These newly synthesised compounds can act as lead molecules for the development of larvicides and antibiotic agents.
Keywords: Antibacterial; Antifungal; DFT; Larvicidal activity; Mannich base; Molecular docking.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Saha N, Wanjari PJ, Dubey G, Mahawar N, Bharatam PV. Metal-free synthesis of imidazoles and 2-aminoimidazoles. J Mol Struct. 2023;15(1272):134092. doi: 10.1016/j.molstruc.2022.134092. - DOI
-
- Deswal L, Verma V, Kumar D, Deswal Y, Kumar A, Kumar R, Parshad M, Bhatia M. Synthesis, antimicrobial and α-glucosidase inhibition of new benzimidazole-1, 2, 3-triazole-indoline derivatives: a combined experimental and computational venture. Chem Pap. 2022;76(12):7607–7622. doi: 10.1007/s11696-022-02436-1. - DOI
-
- Punia S, Verma V, Kumar D, Kumar A, Deswal L, Singh G, Sahoo SC. Pyrazolyl-Imidazole clubbed 1, 2, 3-triazoles: synthesis, structure explication and antimicrobial evaluation. J Mol Struct. 2022;15(1262):133060. doi: 10.1016/j.molstruc.2022.133060. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
