Apomorphine Sublingual Film Compared with Subcutaneous Apomorphine for OFF Episodes in Parkinson's Disease: An Open-Label, Randomized, Crossover Study
- PMID: 37980683
- PMCID: PMC10741320
- DOI: 10.3233/JPD-230072
Apomorphine Sublingual Film Compared with Subcutaneous Apomorphine for OFF Episodes in Parkinson's Disease: An Open-Label, Randomized, Crossover Study
Abstract
Background: Apomorphine sublingual film (SL-APO) and subcutaneous apomorphine (SC-APO) have been used for the treatment of OFF episodes in Parkinson's disease (PD). No study has prospectively compared efficacy and safety of these formulations.
Objective: To compare SL-APO with SC-APO for treatment of OFF episodes in PD.
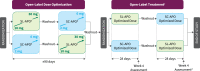
Methods: An open-label, randomized, crossover study assessed SL-APO versus SC-APO in patients with PD and OFF episodes (N = 113). Doses were optimized in randomly assigned order. SL-APO dose initiation (10 mg) occurred in clinic; further dose optimization (15-30 mg; 5-mg increments) occurred primarily at home. SC-APO dosing (2-6 mg; 1-mg increments) occurred entirely in clinic. After a 3-7-day washout, patients were randomized 1 : 1 to 4 weeks of treatment with their optimized dose of SL-APO or SC-APO, followed by washout and 4 weeks of crossover treatment.
Results: Propensity score matching applied on 159 patients (STN-DBS n = 75, MED n = 84) resulted in 40 patients in each treatment group. At 36-month follow-up, STN-DBS led to significantly better PDSS and PDQ-8 change scores, which were significantly correlated. We observed no significant effects for HADS and no significant correlations between change scores in PDSS, HADS, and LEDD.
Conclusions: We report Class IIb evidence of beneficial effects of STN-DBS on quality of sleep at 36-month follow-up, which were associated with QoL improvement independent of depression and dopaminergic medication. Our study highlights the importance of sleep for assessments of DBS outcomes.
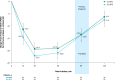
Results: No difference was observed between SL-APO and SC-APO for change from predose to 90 minutes postdose in Movement Disorder Society Unified Parkinson's Disease Rating Scale Part III score at week 4 (primary endpoint), assessed by a blinded rater (-13.6 vs. -13.8, respectively; p = NS). Overall, 72.2% of patients preferred SL-APO compared with SC-APO/no preference (p = 0.0002) per the Treatment Preference Questionnaire (secondary endpoint). Patients reported greater satisfaction with SL-APO compared with SC-APO, per mean scores of convenience (73.7 vs. 53.5) and global satisfaction (63.9 vs. 57.6) on the Treatment Satisfaction Questionnaire for Medication (other endpoint). The safety profiles of both treatments were generally comparable and were well-tolerated.
Conclusions: Patients reported overall preference for and greater satisfaction with SL-APO over SC-APO.
Keywords: Apomorphine sublingual film; OFF episode; Parkinson’s disease; carbidopa/levodopa; subcutaneous apomorphine.
Conflict of interest statement
FS is an Editorial Board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer review and has received grants and/or research support from Zambon and honoraria or consultation fees from Bial, Biogen, Britania Pharmaceuticals, Chiesi, Cynapsus Therapeutics, Kyowa Kirin, Lundbeck, NeuroDerm, Sunovion Pharmaceuticals Inc., and Zambon.
OR has received grants and/or research support from Agence Nationale de la Recherche (ANR), CHU de Toulouse, France-Parkinson, INSERM-DHOS Recherche Clinique Translationnelle, Michael J. Fox Foundation, and Programme Hospitalier de Recherche Clinique, European Commission (FP7, H2020) and honoraria or consultation fees from AbbVie, Adamas, Acorda, Aguettant, Alkahest, AlzProtect, ApoPharma, AstraZeneca, Bial, Biogen, Britannia Pharmaceuticals, Bukwang, Cerevel, Clevexel, Irlab, Eli-Lilly, Lundbeck, NeuroDerm, ONO Pharma, Orion Pharma, Osmotica, Oxford Biomedica, Pfizer, Prexton Therapeutics, Sanofi, Servier, Sunovion Pharmaceuticals Inc., Theranexus, Takeda, Teva Pharmaceuticals, UCB, Watermark Research, XenoPort, XO, and Zambon.
WP has received grants and/or research support from Michael J. Fox Foundation, EU FP7 & Horizon 2020; personal fees from AC Immune, Alterity, AbbVie, Affiris, Bial, Biogen, Britannia Pharmaceuticals, Eli Lilly, Lundbeck, NeuroDerm, Neurocrine, Roche, Sunovion Pharmaceuticals Inc., Stada, Takeda, UCB, and Zambon; and royalties from Cambridge University Press, Oxford University Press, Thieme, and Wiley Blackwell.
KRC is an Editorial Board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer review and has received grants and/or research support from BIAL, EU Horizon 2020, Parkinson’s UK, NIHR, Parkinson’s Foundation, and Wellcome Trust and honoraria or consulting fees from 4D Pharma, AbbVie, Acadia, Bial, Boehringer Ingelheim, Britannia Pharmaceuticals, Cynapsus Therapeutics, GKC, Kyowa Kirin, Lobsor Pharmaceuticals, Novartis, Profile Pharma, Roche, Scion, SK Pharma, Stada, Sunovion Pharmaceuticals Inc., Theravance Biopharma, UCB Pharma, and Zambon.
JK has received honoraria or consultation fees from AbbVie, Bial, Biogen, Desitin, Esteve, Licher MT, Medtronic, NeuroDerm, Novartis, STADA, UCB Pharma, and Zambon; in addition, he is Specialty Chief Editor in
LLM has received compensated advisory services, consulting, research grant support, or speaker honoraria from AbbVie, Bial, Italfarmaco, NeuroDerm, Roche, Stada, Sunovion Pharmaceuticals Inc., UCB Pharma, and Zambon.
YZ, AB, and EP were employees of Sunovion Pharmaceuticals Inc. at the time the analyses were conducted.
SW is an employee of Sumitomo Pharma America, Inc.
Figures

References
-
- Olanow CW, Stern MB, Sethi K (2009) The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 72, S1–S136. - PubMed
-
- Chou KL, Stacy M, Simuni T, Miyasaki J, Oertel WH, Sethi K, Fernandez HH, Stocchi F (2018) The spectrum of “off” in Parkinson’s disease: What have we learned over 40 years? Parkinsonism Relat Disord 51, 9–16. - PubMed
-
- Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: A review. . JAMA 323, 548–560. - PubMed
-
- Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C (2018) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. . Mov Disord 33, 1248–1266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

