Strategies for Development of Synthetic Heart Valve Tissue Engineering Scaffolds
- PMID: 37981978
- PMCID: PMC10655624
- DOI: 10.1016/j.pmatsci.2023.101173
Strategies for Development of Synthetic Heart Valve Tissue Engineering Scaffolds
Abstract
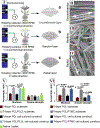
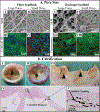
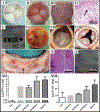
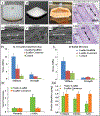
The current clinical solutions, including mechanical and bioprosthetic valves for valvular heart diseases, are plagued by coagulation, calcification, nondurability, and the inability to grow with patients. The tissue engineering approach attempts to resolve these shortcomings by producing heart valve scaffolds that may deliver patients a life-long solution. Heart valve scaffolds serve as a three-dimensional support structure made of biocompatible materials that provide adequate porosity for cell infiltration, and nutrient and waste transport, sponsor cell adhesion, proliferation, and differentiation, and allow for extracellular matrix production that together contributes to the generation of functional neotissue. The foundation of successful heart valve tissue engineering is replicating native heart valve architecture, mechanics, and cellular attributes through appropriate biomaterials and scaffold designs. This article reviews biomaterials, the fabrication of heart valve scaffolds, and their in-vitro and in-vivo evaluations applied for heart valve tissue engineering.
Keywords: Heart valve; fiber; hydrogel; scaffold; solid porous; tissue engineering.
Figures




















Similar articles
-
Modifying decellularized aortic valve scaffolds with stromal cell-derived factor-1α loaded proteolytically degradable hydrogel for recellularization and remodeling.Acta Biomater. 2019 Apr 1;88:280-292. doi: 10.1016/j.actbio.2019.02.002. Epub 2019 Feb 2. Acta Biomater. 2019. PMID: 30721783
-
Strategies for development of decellularized heart valve scaffolds for tissue engineering.Biomaterials. 2022 Sep;288:121675. doi: 10.1016/j.biomaterials.2022.121675. Epub 2022 Jul 18. Biomaterials. 2022. PMID: 35953330 Review.
-
Scaffolds for tissue engineering of cardiac valves.Acta Biomater. 2014 Jul;10(7):2877-93. doi: 10.1016/j.actbio.2014.03.014. Epub 2014 Mar 24. Acta Biomater. 2014. PMID: 24675108 Review.
-
Living nano-micro fibrous woven fabric/hydrogel composite scaffolds for heart valve engineering.Acta Biomater. 2017 Mar 15;51:89-100. doi: 10.1016/j.actbio.2017.01.051. Epub 2017 Jan 18. Acta Biomater. 2017. PMID: 28110071
-
Engineering of a polymer layered bio-hybrid heart valve scaffold.Mater Sci Eng C Mater Biol Appl. 2015 Jun;51:263-73. doi: 10.1016/j.msec.2015.03.009. Epub 2015 Mar 11. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 25842134
Cited by
-
Non-immune factors cause prolonged myofibroblast phenotype in implanted synthetic heart valve scaffolds.Appl Mater Today. 2024 Aug;39:102323. doi: 10.1016/j.apmt.2024.102323. Epub 2024 Jul 16. Appl Mater Today. 2024. PMID: 39131741 Free PMC article.
-
A chronological history of heart valve prostheses to offer perspectives of their limitations.Front Bioeng Biotechnol. 2025 Feb 14;13:1533421. doi: 10.3389/fbioe.2025.1533421. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40028289 Free PMC article. Review.
-
Innovative hydrogel solutions for articular cartilage regeneration: a comprehensive review.Int J Surg. 2024 Dec 1;110(12):7984-8001. doi: 10.1097/JS9.0000000000002076. Int J Surg. 2024. PMID: 39236090 Free PMC article. Review.
-
Mechanistic Insights into Bioprosthetic Heart Valve Calcification and Anti-Calcification Strategies.Rev Cardiovasc Med. 2025 May 20;26(5):36688. doi: 10.31083/RCM36688. eCollection 2025 May. Rev Cardiovasc Med. 2025. PMID: 40475731 Free PMC article. Review.
-
Biodegradable Polyesters: Approaches to Increase Degradation Rates for Biomedical Applications.ACS Macro Lett. 2025 Aug 19;14(8):1221-1240. doi: 10.1021/acsmacrolett.5c00417. Epub 2025 Aug 10. ACS Macro Lett. 2025. PMID: 40783929 Free PMC article. Review.
References
-
- Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, et al. Global epidemiology of valvular heart disease. Nature Reviews Cardiology. 2021;18:853–64 - PubMed
-
- Snyder Y, Jana S. Strategies for development of decellularized heart valve scaffolds for tissue engineering. Biomaterials. 2022;288:121675. - PubMed
-
- Carrel T, Dembitsky WP, de Mol B, Obrist D, Dreyfus G, Meuris B, et al. Non-physiologic closing of bi-leaflet mechanical heart prostheses requires a new tri-leaflet valve design. International journal of cardiology. 2020;304:125–7. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
