Deterministic nuclear reprogramming of mammalian nuclei to a totipotency-like state by Amphibian meiotic oocytes for stem cell therapy in humans
- PMID: 37982514
- PMCID: PMC10924218
- DOI: 10.1242/bio.060011
Deterministic nuclear reprogramming of mammalian nuclei to a totipotency-like state by Amphibian meiotic oocytes for stem cell therapy in humans
Abstract
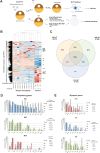
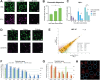
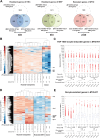
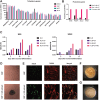
The ultimate aim of nuclear reprogramming is to provide stem cells or differentiated cells from unrelated cell types as a cell source for regenerative medicine. A popular route towards this is transcription factor induction, and an alternative way is an original procedure of transplanting a single somatic cell nucleus to an unfertilized egg. A third route is to transplant hundreds of cell nuclei into the germinal vesicle (GV) of a non-dividing Amphibian meiotic oocyte, which leads to the activation of silent genes in 24 h and robustly induces a totipotency-like state in almost all transplanted cells. We apply this third route for potential therapeutic use and describe a procedure by which the differentiated states of cells can be reversed so that totipotency and pluripotency gene expression are regained. Differentiated cells are exposed to GV extracts and are reprogrammed to form embryoid bodies, which shows the maintenance of stemness and could be induced to follow new directions of differentiation. We conclude that much of the reprogramming effect of eggs is already present in meiotic oocytes and does not require cell division or selection of dividing cells. Reprogrammed cells by oocytes could serve as replacements for defective adult cells in humans.
Keywords: Germinal vesicle; Oocyte; Reprogramming resistant gene; Somatic cell nuclear reprogramming; Stem cell therapy; Totipotency-like stem cell.
© 2024. Published by The Company of Biologists Ltd.
Figures
References
-
- Buganim, Y., Faddah, D. A., Cheng, A. W., Itskovich, E., Markoulaki, S., Ganz, K., Klemm, S. L., Van Oudenaarden, A. and Jaenisch, R. (2012). Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 150, 1209-1222. 10.1016/j.cell.2012.08.023 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

