Klebsiella pneumoniae TolC contributes to antimicrobial resistance, exopolysaccharide production, and virulence
- PMID: 37982617
- PMCID: PMC10715176
- DOI: 10.1128/iai.00303-23
Klebsiella pneumoniae TolC contributes to antimicrobial resistance, exopolysaccharide production, and virulence
Abstract
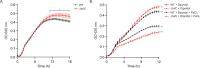
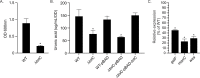
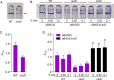
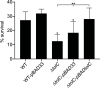
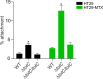
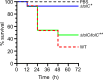
Klebsiella pneumoniae is a Gram-negative bacterium that causes a variety of human diseases, ranging from pneumonia to urinary tract infections and invasive diseases. The emergence of K. pneumoniae strains that are resistant to multiple antibiotics has made treatment more complex and led to K. pneumoniae becoming a global health threat. Addressing this threat necessitates the development of new therapeutic strategies to combat this pathogen, including strategies to overcome antimicrobial resistance and therapeutics for novel targets such as antivirulence. Here, we investigated the function of TolC, an outer membrane protein essential for the function of tripartite transporters, in K. pneumoniae. Mutation of tolC rendered K. pneumoniae hypersensitive to multiple antibiotics. Moreover, the tolC mutation impaired capsule production and affected the expression of key capsule biosynthetic genes, indicating a regulatory role for TolC in capsule biosynthesis. Additionally, TolC was essential for growth under iron-limiting conditions, suggesting its involvement in iron acquisition. The tolC mutant exhibited increased adherence to human enterocytes and enhanced serum sensitivity. In the Galleria mellonella infection model, the tolC mutant displayed reduced virulence compared to the wild type. Our findings highlight the pleiotropic role of TolC in K. pneumoniae pathobiology, influencing antimicrobial resistance, capsule production, iron homeostasis, adherence to host cells, and virulence. Understanding the multifaceted role of TolC in K. pneumoniae may guide the development of new therapeutic strategies against this pathogen. .
Keywords: Klebsiella pneumoniae; antimicrobial resistance; capsule; hypermucoviscosity; pathogenesis; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Klebsiella pneumoniae Regulatory Mutant Has Reduced Capsule Expression but Retains Hypermucoviscosity.mBio. 2019 Mar 26;10(2):e00089-19. doi: 10.1128/mBio.00089-19. mBio. 2019. PMID: 30914502 Free PMC article.
-
Identification of Two Regulators of Virulence That Are Conserved in Klebsiella pneumoniae Classical and Hypervirulent Strains.mBio. 2018 Aug 7;9(4):e01443-18. doi: 10.1128/mBio.01443-18. mBio. 2018. PMID: 30087173 Free PMC article.
-
Urine-mediated suppression of Klebsiella pneumoniae mucoidy is counteracted by spontaneous Wzc variants altering capsule chain length.mSphere. 2023 Oct 24;8(5):e0028823. doi: 10.1128/msphere.00288-23. Epub 2023 Aug 23. mSphere. 2023. PMID: 37610214 Free PMC article.
-
The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella pneumoniae.Int J Environ Res Public Health. 2020 Aug 28;17(17):6278. doi: 10.3390/ijerph17176278. Int J Environ Res Public Health. 2020. PMID: 32872324 Free PMC article. Review.
-
Potential therapeutic targets of Klebsiella pneumoniae: a multi-omics review perspective.Brief Funct Genomics. 2022 Apr 11;21(2):63-77. doi: 10.1093/bfgp/elab038. Brief Funct Genomics. 2022. PMID: 34448478 Review.
Cited by
-
Biofilm Formation, Antibiotic Resistance, and Virulence Analysis of Human and Avian Origin Klebsiella pneumoniae from Jiangsu, China.Vet Sci. 2025 Jun 30;12(7):628. doi: 10.3390/vetsci12070628. Vet Sci. 2025. PMID: 40711288 Free PMC article.
-
Klebsiella pneumoniae capsular polysaccharide: Mechanism in regulation of synthesis, virulence, and pathogenicity.Virulence. 2024 Dec;15(1):2439509. doi: 10.1080/21505594.2024.2439509. Epub 2024 Dec 13. Virulence. 2024. PMID: 39668724 Free PMC article. Review.
-
Shedding light on Klebsiella pneumoniae virulence: Engineering of broad host range bioluminescence reporter vectors for transcriptional analysis in drug resistant pathogens.Plasmid. 2024 Sep-Nov;131-132:102734. doi: 10.1016/j.plasmid.2024.102734. Epub 2024 Oct 29. Plasmid. 2024. PMID: 39481464 Free PMC article.
-
A historical perspective on the multifunctional outer membrane channel protein TolC in Escherichia coli.NPJ Antimicrob Resist. 2025 Jan 25;3(1):6. doi: 10.1038/s44259-025-00078-3. NPJ Antimicrob Resist. 2025. PMID: 39863731 Free PMC article. Review.
-
TolC facilitates the intracellular survival and immunomodulation of Salmonella Typhi in human host cells.Virulence. 2024 Dec;15(1):2395831. doi: 10.1080/21505594.2024.2395831. Epub 2024 Sep 9. Virulence. 2024. PMID: 39185619 Free PMC article.
References
-
- Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D, Jenney A, Connor TR, Hsu LY, Severin J, et al. . 2015. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci USA 112:E3574–E3581. doi:10.1073/pnas.1501049112 - DOI - PMC - PubMed
-
- Wyres KL, Wick RR, Judd LM, Froumine R, Tokolyi A, Gorrie CL, Lam MMC, Duchêne S, Jenney A, Holt KE. 2019. Distinct evolutionary dynamics of horizontal gene transfer in drug resistant and virulent clones of Klebsiella pneumoniae. PLoS Genet 15:e1008114. doi:10.1371/journal.pgen.1008114 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

