Chimeric antigen receptor T cell therapy: a new emerging landscape in autoimmune rheumatic diseases
- PMID: 37982747
- PMCID: PMC11065442
- DOI: 10.1093/rheumatology/kead616
Chimeric antigen receptor T cell therapy: a new emerging landscape in autoimmune rheumatic diseases
Abstract
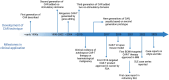
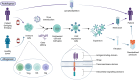
Chimeric antigen receptor T cell (CAR-T) therapy, an innovative immune cell therapy, has revolutionized the treatment landscape of haematological malignancies. The past 2 years has witnessed the successful application of CD19-targeting CAR constructs in refractory cases of autoimmune rheumatic diseases, including systemic lupus erythematosus, systemic sclerosis and anti-synthetase syndrome. In comparison with existing B cell depletion therapies, targeting CD19 has demonstrated a more rapid and profound therapeutic effect, enabling drug-free remission with manageable adverse events. These promising results necessitate validation through long-term, large-sample randomized controlled studies. Corroborating the role of CAR-T therapy in refractory rheumatological disorders and affirming safety, efficacy and durability of responses are the aims of future clinical studies. Optimizing the engineering strategies and better patient selection are also critical to further refining the successful clinical implementation of CAR-T therapy.
Keywords: CAR-T; autoimmune rheumatic diseases; immunotherapy; lupus; myositis; scleroderma.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
References
-
- Lee DW, Santomasso BD, Locke FL. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–38. - PubMed
-
- Schuster SJ, Bishop MR, Tam CS. et al.; JULIET Investigators . Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

