Safety and Efficacy of Adeno-Associated Viral Gene Therapy in Patients With Retinal Degeneration: A Systematic Review and Meta-Analysis
- PMID: 37982768
- PMCID: PMC10668613
- DOI: 10.1167/tvst.12.11.24
Safety and Efficacy of Adeno-Associated Viral Gene Therapy in Patients With Retinal Degeneration: A Systematic Review and Meta-Analysis
Abstract
Purpose: This systematic review evaluates the safety and efficacy of ocular gene therapy using adeno-associated virus (AAV).
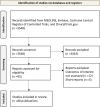
Methods: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov were searched systematically for controlled or non-controlled interventional gene therapy studies using key words related to retinal diseases, gene therapy, and AAV vectors. The primary outcome measure was safety, based on ocular severe adverse events (SAEs). Secondary outcome measures evaluated efficacy of the therapy based on best corrected visual acuity (BCVA) and improvements in visual sensitivity and systemic involvement following ocular delivery. Pooling was done using a DerSimonian Laird random effects model. Risk of bias was assessed using the Cochrane Risk of Bias Tool, version 1.
Results: Our search identified 3548 records. Of these, 80 publications met eligibility criteria, representing 28 registered clinical trials and 5 postmarket surveillance studies involving AAV gene therapy for Leber congenital amaurosis (LCA), choroideremia, Leber hereditary optic neuropathy (LHON), age-related macular degeneration (AMD), retinitis pigmentosa (RP), X-linked retinoschisis, and achromatopsia. Overall, AAV therapy vectors were associated with a cumulative incidence of at least one SAE of 8% (95% confidence intervals [CIs] of 5% to 12%). SAEs were often associated with the surgical procedure rather than the therapeutic vector itself. Poor or inconsistent reporting of adverse events (AEs) were a limitation for the meta-analysis. The proportion of patients with any improvement in BCVA and visual sensitivity was 41% (95% CIs of 31% to 51%) and 51% (95% CIs of 31% to 70%), respectively. Systemic immune involvement was associated with a cumulative incidence of 31% (95% CI = 21% to 42%).
Conclusions: AAV gene therapy vectors appear to be safe but the surgical procedure required to deliver them is associated with some risk. The large variability in efficacy can be attributed to the small number of patients treated, the heterogeneity of the population and the variability in dosage, volume, and follow-up.
Translational relevance: This systematic review will help to inform and guide future clinical trials.
Conflict of interest statement
Disclosure:
Figures




References
-
- Wong WL, Su X, Li X, et al. .. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2: e106–e116. - PubMed
-
- Broadgate S, Yu J, Downes SM, Halford S.. Unravelling the genetics of inherited retinal dystrophies: past, present and future. Prog Retin Eye Res. 2017; 59: 53–96. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

