Niosomal formulation of mefenamic acid for enhanced cancer targeting; preparation, characterization and biodistribution study using radiolabeling technique
- PMID: 37982828
- PMCID: PMC10725351
- DOI: 10.1007/s00432-023-05482-8
Niosomal formulation of mefenamic acid for enhanced cancer targeting; preparation, characterization and biodistribution study using radiolabeling technique
Abstract
Background: This work aimed to prepare niosomal formulations of an anticancer agent [mefenamic acid (MEF)] to enhance its cancer targeting. 131I was utilized as a radiolabeling isotope to study the radio-kinetics of MEF niosomes.
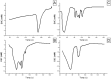
Methods: niosomal formulations were prepared by the ether injection method and assessed for entrapment efficiency (EE%), zeta potential (ZP), polydispersity index (PDI) and particle size (PS). MEF was labeled with 131I by direct electrophilic substitution reaction through optimization of radiolabeling-related parameters. In the radio-kinetic study, the optimal 131I-MEF niosomal formula was administered intravenously (I.V.) to solid tumor-bearing mice and compared to I.V. 131I-MEF solution as a control.
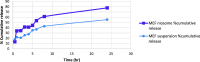
Results: the average PS and ZP values of the optimal formulation were 247.23 ± 2.32 nm and - 28.3 ± 1.21, respectively. The highest 131I-MEF labeling yield was 98.7 ± 0.8%. The biodistribution study revealed that the highest tumor uptake of 131I-MEF niosomal formula and 131I-MEF solution at 60 min post-injection were 2.73 and 1.94% ID/g, respectively.
Conclusion: MEF-loaded niosomes could be a hopeful candidate in cancer treatment due to their potent tumor uptake. Such high targeting was attributed to passive targeting of the nanosized niosomes and confirmed by radiokinetic evaluation.
Keywords: 131I; Anticancer; Mefenamic acid; Niosomes; Radiokinetics.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Abbas Z, Rehman SJN (2018) An overview of cancer treatment modalities. Neoplasm 1:139–157
-
- Aboumanei MH, Mahmoud AF (2022) Development of tamoxifen in situ gel nanoemulsion for ocular delivery in photoreceptor degeneration disorder: in vitro characterization, 131I-Radiolabeling, and in vivo biodistribution studies. J Pharm Innov 18:369–380 - DOI
-
- Ahmed AM et al (2013) The application of Plackett-Burman design and response surface methodology for optimization of formulation variables to produce Piroxicam niosomes. Int J Drug Dev Res 5(2):121–130
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

