Mechanism of stepwise electron transfer in six-transmembrane epithelial antigen of the prostate (STEAP) 1 and 2
- PMID: 37983176
- PMCID: PMC10659578
- DOI: 10.7554/eLife.88299
Mechanism of stepwise electron transfer in six-transmembrane epithelial antigen of the prostate (STEAP) 1 and 2
Abstract
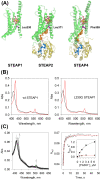
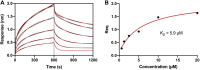
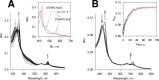
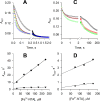
Six transmembrane epithelial antigen of the prostate (STEAP) 1-4 are membrane-embedded hemoproteins that chelate a heme prosthetic group in a transmembrane domain (TMD). STEAP2-4, but not STEAP1, have an intracellular oxidoreductase domain (OxRD) and can mediate cross-membrane electron transfer from NADPH via FAD and heme. However, it is unknown whether STEAP1 can establish a physiologically relevant electron transfer chain. Here, we show that STEAP1 can be reduced by reduced FAD or soluble cytochrome b5 reductase that serves as a surrogate OxRD, providing the first evidence that STEAP1 can support a cross-membrane electron transfer chain. It is not clear whether FAD, which relays electrons from NADPH in OxRD to heme in TMD, remains constantly bound to the STEAPs. We found that FAD reduced by STEAP2 can be utilized by STEAP1, suggesting that FAD is diffusible rather than staying bound to STEAP2. We determined the structure of human STEAP2 in complex with NADP+ and FAD to an overall resolution of 3.2 Å by cryo-electron microscopy and found that the two cofactors bind STEAP2 similarly as in STEAP4, suggesting that a diffusible FAD is a general feature of the electron transfer mechanism in the STEAPs. We also demonstrated that STEAP2 reduces ferric nitrilotriacetic acid (Fe3+-NTA) significantly slower than STEAP1 and proposed that the slower reduction is due to the poor Fe3+-NTA binding to the highly flexible extracellular region in STEAP2. These results establish a solid foundation for understanding the function and mechanisms of the STEAPs.
Keywords: STEAP; biochemistry; chemical biology; electron transfer; enzyme mechanism; human; membrane-bound hemoprotein.
© 2023, Chen, Wang et al.
Conflict of interest statement
KC, LW, JS, AT, MZ, GW No competing interests declared
Figures









Update of
- doi: 10.1101/2021.12.23.474010
- doi: 10.7554/eLife.88299.1
- doi: 10.7554/eLife.88299.2
References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica. Section D, Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Chen S, McMullan G, Faruqi AR, Murshudov GN, Short JM, Scheres SHW, Henderson R. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy. 2013;135:24–35. doi: 10.1016/j.ultramic.2013.06.004. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

