Differential vascular endothelial cell toxicity of established and novel BCR-ABL tyrosine kinase inhibitors
- PMID: 37983208
- PMCID: PMC10659179
- DOI: 10.1371/journal.pone.0294438
Differential vascular endothelial cell toxicity of established and novel BCR-ABL tyrosine kinase inhibitors
Abstract
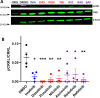
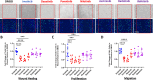
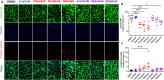
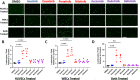
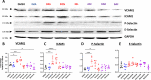
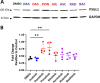
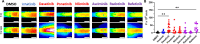
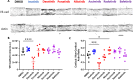
BCR-ABL tyrosine kinase inhibitors (TKIs) have dramatically improved survival in Philadelphia chromosome-positive leukemias. Newer BCR-ABL TKIs provide superior cancer outcomes but with increased risk of acute arterial thrombosis, which further increases in patients with cardiovascular comorbidities and mitigates survival benefits compared to imatinib. Recent studies implicate endothelial cell (EC) damage in this toxicity by unknown mechanisms with few side-by-side comparisons of multiple TKIs and with no available data on endothelial impact of recently approved TKIs or novels TKIs being tested in clinical trials. To characterize BCR-ABL TKI induced EC dysfunction we exposed primary human umbilical vein ECs in 2D and 3D culture to clinically relevant concentrations of seven BCR-ABL TKIs and quantified their impact on EC scratch-wound healing, viability, inflammation, and permeability mechanisms. Dasatinib, ponatinib, and nilotinib, the TKIs associated with thrombosis in patients, all significantly impaired EC wound healing, survival, and proliferation compared to imatinib, but only dasatinib and ponatinib impaired cell migration and only nilotinib enhanced EC necrosis. Dasatinib and ponatinib increased leukocyte adhesion to ECs with upregulation of adhesion molecule expression in ECs (ICAM1, VCAM1, and P-selectin) and leukocytes (PSGL1). Dasatinib increased permeability and impaired cell junctional integrity in human engineered microvessels, consistent with its unique association with pleural effusions. Of the new agents, bafetinib decreased EC viability and increased microvessel permeability while asciminib and radotinib did not impact any EC function tested. In summary, the vasculotoxic TKIs (dasatinib, ponatinib, nilotinib) cause EC toxicity but with mechanistic differences, supporting the potential need for drug-specific vasculoprotective strategies. Asciminib and radotinib do not induce EC toxicity at clinically relevant concentrations suggesting a better safety profile.
Copyright: © 2023 Wang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- SEER CML Facts [Internet].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

