Voltage gated sodium and calcium channels: Discovery, structure, function, and Pharmacology
- PMID: 37983307
- PMCID: PMC10761118
- DOI: 10.1080/19336950.2023.2281714
Voltage gated sodium and calcium channels: Discovery, structure, function, and Pharmacology
Abstract
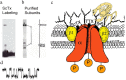
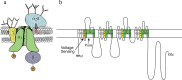
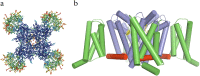
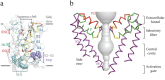
Voltage-gated sodium channels initiate action potentials in nerve and muscle, and voltage-gated calcium channels couple depolarization of the plasma membrane to intracellular events such as secretion, contraction, synaptic transmission, and gene expression. In this Review and Perspective article, I summarize early work that led to identification, purification, functional reconstitution, and determination of the amino acid sequence of the protein subunits of sodium and calcium channels and showed that their pore-forming subunits are closely related. Decades of study by antibody mapping, site-directed mutagenesis, and electrophysiological recording led to detailed two-dimensional structure-function maps of the amino acid residues involved in voltage-dependent activation and inactivation, ion permeation and selectivity, and pharmacological modulation. Most recently, high-resolution three-dimensional structure determination by X-ray crystallography and cryogenic electron microscopy has revealed the structural basis for sodium and calcium channel function and pharmacological modulation at the atomic level. These studies now define the chemical basis for electrical signaling and provide templates for future development of new therapeutic agents for a range of neurological and cardiovascular diseases.
Keywords: X-ray crystallography; calcium channel; cryogenic electron microscopy; protein structure; sodium channel.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures






References
-
- Hille B. Ionic channels of excitable membranes. 3rd ed. Sunderland (MA): Sinauer Associates Inc.; 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
