Growth hormone-releasing hormone receptor antagonist MIA-602 attenuates cardiopulmonary injury induced by BSL-2 rVSV-SARS-CoV-2 in hACE2 mice
- PMID: 37983492
- PMCID: PMC10691341
- DOI: 10.1073/pnas.2308342120
Growth hormone-releasing hormone receptor antagonist MIA-602 attenuates cardiopulmonary injury induced by BSL-2 rVSV-SARS-CoV-2 in hACE2 mice
Abstract
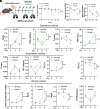
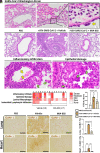
COVID-19 pneumonia causes acute lung injury and acute respiratory distress syndrome (ALI/ARDS) characterized by early pulmonary endothelial and epithelial injuries with altered pulmonary diffusing capacity and obstructive or restrictive physiology. Growth hormone-releasing hormone receptor (GHRH-R) is expressed in the lung and heart. GHRH-R antagonist, MIA-602, has been reported to modulate immune responses to bleomycin lung injury and inflammation in granulomatous sarcoidosis. We hypothesized that MIA-602 would attenuate rVSV-SARS-CoV-2-induced pulmonary dysfunction and heart injury in a BSL-2 mouse model. Male and female K18-hACE2tg mice were inoculated with SARS-CoV-2/USA-WA1/2020, BSL-2-compliant recombinant VSV-eGFP-SARS-CoV-2-Spike (rVSV-SARS-CoV-2), or PBS, and lung viral load, weight loss, histopathology, and gene expression were compared. K18-hACE2tg mice infected with rVSV-SARS-CoV-2 were treated daily with subcutaneous MIA-602 or vehicle and conscious, unrestrained plethysmography performed on days 0, 3, and 5 (n = 7 to 8). Five days after infection mice were killed, and blood and tissues collected for histopathology and protein/gene expression. Both native SARS-CoV-2 and rVSV-SARS-CoV-2 presented similar patterns of weight loss, infectivity (~60%), and histopathologic changes. Daily treatment with MIA-602 conferred weight recovery, reduced lung perivascular inflammation/pneumonia, and decreased lung/heart ICAM-1 expression compared to vehicle. MIA-602 rescued altered respiratory rate, increased expiratory parameters (Te, PEF, EEP), and normalized airflow parameters (Penh and Rpef) compared to vehicle, consistent with decreased airway inflammation. RNASeq followed by protein analysis revealed heightened levels of inflammation and end-stage necroptosis markers, including ZBP1 and pMLKL induced by rVSV-SARS-CoV-2, that were normalized by MIA-602 treatment, consistent with an anti-inflammatory and pro-survival mechanism of action in this preclinical model of COVID-19 pneumonia.
Keywords: ARDS; COVID-19; GHRH-R; Necroptosis; SARS-CoV-2.
Conflict of interest statement
Competing interests statement:A.V.S. and R.M.J. are listed as co-inventors on patents of GHRH analogs, which were assigned to the University of Miami and Veterans Affairs Department.
Figures


Similar articles
-
BSL2-compliant lethal mouse model of SARS-CoV-2 and variants of concern to evaluate therapeutics targeting the Spike protein.Front Immunol. 2022 Jul 28;13:919815. doi: 10.3389/fimmu.2022.919815. eCollection 2022. Front Immunol. 2022. PMID: 35967447 Free PMC article.
-
Growth hormone-releasing hormone antagonist MIA-602 inhibits inflammation induced by SARS-CoV-2 spike protein and bacterial lipopolysaccharide synergism in macrophages and human peripheral blood mononuclear cells.Front Immunol. 2023 Aug 15;14:1231363. doi: 10.3389/fimmu.2023.1231363. eCollection 2023. Front Immunol. 2023. PMID: 37649486 Free PMC article.
-
Growth Hormone-Releasing Hormone Receptor Antagonist Modulates Lung Inflammation and Fibrosis due to Bleomycin.Lung. 2019 Oct;197(5):541-549. doi: 10.1007/s00408-019-00257-w. Epub 2019 Aug 7. Lung. 2019. PMID: 31392398 Free PMC article.
-
Angiotensin-Converting Enzyme 2 (ACE2) in the Pathogenesis of ARDS in COVID-19.Front Immunol. 2021 Dec 22;12:732690. doi: 10.3389/fimmu.2021.732690. eCollection 2021. Front Immunol. 2021. PMID: 35003058 Free PMC article. Review.
-
The Role of Macrophages in the Pathogenesis of SARS-CoV-2-Associated Acute Respiratory Distress Syndrome.Front Immunol. 2021 May 10;12:682871. doi: 10.3389/fimmu.2021.682871. eCollection 2021. Front Immunol. 2021. PMID: 34040616 Free PMC article. Review.
Cited by
-
Growth hormone-releasing hormone signaling and manifestations within the cardiovascular system.Rev Endocr Metab Disord. 2025 Jun;26(3):397-412. doi: 10.1007/s11154-024-09939-0. Epub 2025 Jan 30. Rev Endocr Metab Disord. 2025. PMID: 39883351 Free PMC article. Review.
-
Efficacy of EA575 as an Antitussive and Mucoactive Agent in Preclinical In Vivo Models.Biomedicines. 2025 Jul 8;13(7):1673. doi: 10.3390/biomedicines13071673. Biomedicines. 2025. PMID: 40722746 Free PMC article.
-
A rat model establishment of bronchopulmonary dysplasia-related lung & brain injury within 28 days after birth.BMC Neurosci. 2024 Nov 28;25(1):73. doi: 10.1186/s12868-024-00912-w. BMC Neurosci. 2024. PMID: 39609737 Free PMC article.
-
Effects of GHRH and its analogues on the Vascular System.Rev Endocr Metab Disord. 2025 Jun;26(3):493-505. doi: 10.1007/s11154-024-09932-7. Epub 2024 Nov 21. Rev Endocr Metab Disord. 2025. PMID: 39570567 Review.
-
Growth hormone - releasing hormone in the immune system.Rev Endocr Metab Disord. 2025 Jun;26(3):457-466. doi: 10.1007/s11154-024-09913-w. Epub 2024 Oct 7. Rev Endocr Metab Disord. 2025. PMID: 39370499 Free PMC article. Review.
References
-
- Lovato A., de Filippis C., Clinical presentation of COVID-19: A systematic review focusing on upper airway symptoms. Ear Nose Throat J. 99, 569–576 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

