Riding apoptotic bodies for cell-cell transmission by African swine fever virus
- PMID: 37983498
- PMCID: PMC10691326
- DOI: 10.1073/pnas.2309506120
Riding apoptotic bodies for cell-cell transmission by African swine fever virus
Abstract
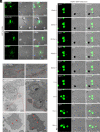
African swine fever virus (ASFV), a devastating pathogen to the worldwide swine industry, mainly targets macrophage/monocyte lineage, but how the virus enters host cells has remained unclear. Here, we report that ASFV utilizes apoptotic bodies (ApoBDs) for infection and cell-cell transmission. We show that ASFV induces cell apoptosis of primary porcine alveolar macrophages (PAMs) at the late stage of infection to productively shed ApoBDs that are subsequently swallowed by neighboring PAMs to initiate a secondary infection as evidenced by electron microscopy and live-cell imaging. Interestingly, the virions loaded within ApoBDs are exclusively single-enveloped particles that are devoid of the outer layer of membrane and represent a predominant form produced during late infection. The in vitro purified ApoBD vesicles are capable of mediating virus infection of naive PAMs, but the transmission can be significantly inhibited by blocking the "eat-me" signal phosphatidyserine on the surface of ApoBDs via Annexin V or the efferocytosis receptor TIM4 on the recipient PAMs via anti-TIM4 antibody, whereas overexpression of TIM4 enhances virus infection. The same treatment however did not affect the infection by intracellular viruses. Importantly, the swine sera to ASFV exert no effect on the ApoBD-mediated transmission but can partially act on the virions lacking the outer layer of membrane. Thus, ASFV has evolved to hijack a normal cellular pathway for cell-cell spread to evade host responses.
Keywords: ASFV; apoptotic bodies; cell to cell transmission; immune evasion.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Similar articles
-
African swine fever virus infection enhances CD14-dependent phagocytosis of porcine alveolar macrophages to promote bacterial uptake and apoptotic body-mediated viral transmission.J Virol. 2025 Jul 22;99(7):e0069025. doi: 10.1128/jvi.00690-25. Epub 2025 Jun 12. J Virol. 2025. PMID: 40503879 Free PMC article.
-
Deletion of the H240R Gene of African Swine Fever Virus Decreases Infectious Progeny Virus Production Due to Aberrant Virion Morphogenesis and Enhances Inflammatory Cytokine Expression in Porcine Macrophages.J Virol. 2022 Feb 9;96(3):e0166721. doi: 10.1128/JVI.01667-21. Epub 2021 Nov 17. J Virol. 2022. PMID: 34787458 Free PMC article.
-
African Swine Fever Virus E120R Protein Inhibits Interferon Beta Production by Interacting with IRF3 To Block Its Activation.J Virol. 2021 Aug 25;95(18):e0082421. doi: 10.1128/JVI.00824-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34190598 Free PMC article.
-
Infection, modulation and responses of antigen-presenting cells to African swine fever viruses.Virus Res. 2018 Oct 15;258:73-80. doi: 10.1016/j.virusres.2018.10.007. Epub 2018 Oct 11. Virus Res. 2018. PMID: 30316802 Review.
-
Structure of African Swine Fever Virus and Associated Molecular Mechanisms Underlying Infection and Immunosuppression: A Review.Front Immunol. 2021 Sep 6;12:715582. doi: 10.3389/fimmu.2021.715582. eCollection 2021. Front Immunol. 2021. PMID: 34552586 Free PMC article. Review.
Cited by
-
Insights and progress on epidemic characteristics, pathogenesis, and preventive measures of African swine fever virus: A review.Virulence. 2025 Dec;16(1):2457949. doi: 10.1080/21505594.2025.2457949. Epub 2025 Mar 6. Virulence. 2025. PMID: 39937724 Free PMC article. Review.
-
Integration of CRISPR screening and proteomic analysis of WDR91 manipulation of endosome-to-cytosol transport of African swine fever virus.Sci China Life Sci. 2025 Jun;68(6):1839-1842. doi: 10.1007/s11427-024-2739-9. Epub 2025 Feb 13. Sci China Life Sci. 2025. PMID: 39960627 No abstract available.
-
Epidemiology, pathogenesis, immune evasion mechanism and vaccine development of porcine Deltacoronavirus.Funct Integr Genomics. 2024 Apr 24;24(3):79. doi: 10.1007/s10142-024-01346-7. Funct Integr Genomics. 2024. PMID: 38653845 Review.
-
The African swine fever virus MGF360-16R protein functions as a mitochondrial-dependent apoptosis inducer by competing with BAX to bind to the HSP60 protein.J Virol. 2025 Apr 15;99(4):e0140124. doi: 10.1128/jvi.01401-24. Epub 2025 Feb 27. J Virol. 2025. PMID: 40013993 Free PMC article.
-
African swine fever virus infection of porcine peripheral blood monocyte-derived macrophages induces the formation of tunneling nanotube-connected large vesicle-like cell segments: a potential mechanism for intercellular ASFV trafficking.Vet Res. 2025 Jul 10;56(1):148. doi: 10.1186/s13567-025-01582-0. Vet Res. 2025. PMID: 40640946 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

