Targeting the Mitochondrial Chaperone TRAP1 Alleviates Vascular Pathologies in Ischemic Retinopathy
- PMID: 37983591
- PMCID: PMC10787068
- DOI: 10.1002/advs.202302776
Targeting the Mitochondrial Chaperone TRAP1 Alleviates Vascular Pathologies in Ischemic Retinopathy
Abstract
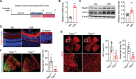
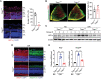
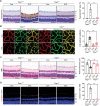
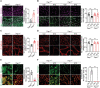
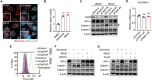
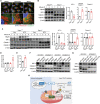
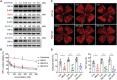
Activation of hypoxia-inducible factor 1α (HIF1α) contributes to blood-retinal barrier (BRB) breakdown and pathological neovascularization responsible for vision loss in ischemic retinal diseases. During disease progression, mitochondrial biology is altered to adapt to the ischemic environment created by initial vascular dysfunction, but the mitochondrial adaptive mechanisms, which ultimately contribute to the pathogenesis of ischemic retinopathy, remain incompletely understood. In the present study, it is identified that expression of mitochondrial chaperone tumor necrosis factor receptor-associated protein 1 (TRAP1) is essential for BRB breakdown and pathologic retinal neovascularization in mouse models mimicking ischemic retinopathies. Genetic Trap1 ablation or treatment with small molecule TRAP1 inhibitors, such as mitoquinone (MitoQ) and SB-U015, alleviate retinal pathologies via proteolytic HIF1α degradation, which is mediated by opening of the mitochondrial permeability transition pore and activation of calcium-dependent protease calpain-1. These findings suggest that TRAP1 can be a promising target for the development of new treatments against ischemic retinopathy, such as retinopathy of prematurity and proliferative diabetic retinopathy.
Keywords: calcium; calpain-1; hypoxia-inducible factor 1α (HIF1α); ischemic retinopathy; mitochondrial permeability transition pore (mPTP); tumor necrosis factor receptor-associated protein 1 (TRAP1).
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
B.H.K. is the founder of SmartinBio. Inc. Other authors declare that they have no competing interests.
Figures
References
-
- Suomalainen A., Battersby B. J., Nat. Rev. Mol. Cell Biol. 2018, 19, 77. - PubMed
-
- a) Rasola A., Neckers L., Picard D., Trends Cell Biol. 2014, 24, 455. - PMC - PubMed
- b) Kang B. H., Plescia J., Dohi T., Rosa J., Doxsey S. J., Altieri D. C., Cell 2007, 131, 257. - PubMed
- c) Felts S. J., Owen B. A. L., Nguyen P., Trepel J., Donner D. B., Toft D. O., J. Biol. Chem. 2000, 275, 3305. - PubMed
-
- Casson R. J., Chidlow G., Crowston J. G., Williams P. A., Wood J. P. M., Prog. Retin. Eye Res. 2021, 81, 100881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022R1C1C2010406/National Research Foundation of Korea
- 2019R1A2C1084371/National Research Foundation of Korea
- 2019R1A2C2086618/National Research Foundation of Korea
- 2018R1A5A1024340/National Research Foundation of Korea
- 2019M3A9A8065669/National Research Foundation of Korea
- 2022M3E5F2017408/National Research Foundation of Korea
- RS-2021-DD120784(HN21C0923000021)/Korea Drug Development Fund
- RS-2021-DD120712(HN21C0882)/Korea Drug Development Fund
- HR22C1832/Ministry of Health and Welfare
- IITP-2023-2020-0-01808/Institute for Information and communications Technology Promotion
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
