Lactate produced by alveolar type II cells suppresses inflammatory alveolar macrophages in acute lung injury
- PMID: 37983890
- PMCID: PMC10914122
- DOI: 10.1096/fj.202301722R
Lactate produced by alveolar type II cells suppresses inflammatory alveolar macrophages in acute lung injury
Abstract
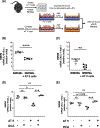
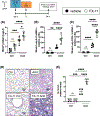
Alveolar inflammation is a hallmark of acute lung injury (ALI), and its clinical correlate is acute respiratory distress syndrome-and it is as a result of interactions between alveolar type II cells (ATII) and alveolar macrophages (AM). In the setting of acute injury, the microenvironment of the intra-alveolar space is determined in part by metabolites and cytokines and is known to shape the AM phenotype. In response to ALI, increased glycolysis is observed in AT II cells, mediated by the transcription factor hypoxia-inducible factor (HIF) 1α, which has been shown to decrease inflammation. We hypothesized that in acute lung injury, lactate, the end product of glycolysis, produced by ATII cells shifts AMs toward an anti-inflammatory phenotype, thus mitigating ALI. We found that local intratracheal delivery of lactate improved ALI in two different mouse models. Lactate shifted cytokine expression of murine AMs toward increased IL-10, while decreasing IL-1 and IL-6 expression. Mice with ATII-specific deletion of Hif1a and mice treated with an inhibitor of lactate dehydrogenase displayed exacerbated ALI and increased inflammation with decreased levels of lactate in the bronchoalveolar lavage fluid; however, all those parameters improved with intratracheal lactate. When exposed to LPS (to recapitulate an inflammatory stimulus as it occurs in ALI), human primary AMs co-cultured with alveolar epithelial cells had reduced inflammatory responses. Taken together, these studies reveal an innate protective pathway, in which lactate produced by ATII cells shifts AMs toward an anti-inflammatory phenotype and dampens excessive inflammation in ALI.
Keywords: acute lung injury; acute respiratory distress syndrome; alveolar epithelium; alveolar macrophage; glycolysis; hypoxia-inducible factor; lactate.
© 2023 Federation of American Societies for Experimental Biology.
Conflict of interest statement
DISCLOSURES
The authors declare no conflicts of interest.
Figures




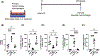
References
-
- Elks PM, van Eeden FJ, Dixon G, et al. Activation of hypoxia-inducible factor-1alpha (Hif-1alpha) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood 2011;118:712–722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

