Polycationic Ru(II) Luminophores: Syntheses, Photophysics, and Application in Electrostatically Driven Sensitization of Lanthanide Luminescence
- PMID: 37984058
- PMCID: PMC10698718
- DOI: 10.1021/acs.inorgchem.3c02352
Polycationic Ru(II) Luminophores: Syntheses, Photophysics, and Application in Electrostatically Driven Sensitization of Lanthanide Luminescence
Abstract
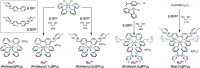
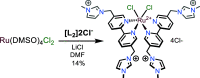
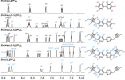
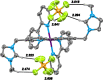
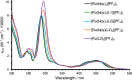
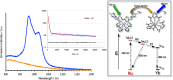
A series of photoluminescent Ru(II) polypyridine complexes have been synthesized in a manner that varies the extent of the cationic charge. Two ligand systems (L1 and L2), based upon 2,2'-bipyridine (bipy) mono- or difunctionalized at the 5- or 5,5'-positions using N-methylimidazolium groups, were utilized. The resulting Ru(II) species therefore carried +3, +4, +6, and +8 complex moieties based on a [Ru(bipy)3]2+ core. Tetra-cationic [Ru(bipy)2(L2)][PF6]4 was characterized using XRD, revealing H-bonding interactions between two of the counteranions and the cationic unit. The ground-state features of the complexes were found to closely resemble those of the parent unfunctionalized [Ru(bipy)3]2+ complex. In contrast, the excited state properties produce a variation in emission maxima, including a bathochromic 44 nm shift of the 3MLCT band for the tetra-cationic complex; interestingly, further increases in overall charge to +6 and +8 produced a hypsochromic shift in the 3MLCT band. Supporting DFT calculations suggest that the trend in emission behavior may, in part, be due to the precise nature of the LUMO and its localization. The utility of a photoactive polycationic Ru(II) complex was then demonstrated through the sensitization of a polyanionic Yb(III) complex in free solution. The study shows that electrostatically driven ion pairing is sufficient to facilitate energy transfer between the 3MLCT donor state of the Ru(II) complex and the accepting 2F5/2 excited state of Yb(III).
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Yong C.-K.; Parkinson P.; Kondratuk D. V.; Chen W.-H.; Stannard A.; Summerfield A.; Sprafke J. K.; O’Sullivan M. C.; Beton P. H.; Anderson H. L.; Herz L. M. Ultrafast Delocalization of Excitation in Synthetic Light-Harvesting Nanorings. Chem. Sci. 2015, 6, 181–189. 10.1039/C4SC02424A. - DOI - PMC - PubMed
-
- Cesana P. T.; Li B. X.; Shepard S. G.; Ting S. I.; Hart S. M.; Olson C. M.; Martinez Alvarado J. I.; Son M.; Steiman T. J.; Castellano F. N.; Doyle A. G.; MacMillan D. W. C.; Schlau-Cohen G. S. A Biohybrid Strategy for Enabling Photoredox Catalysis with Low-Energy Light. Chem 2022, 8, 174–185. 10.1016/j.chempr.2021.10.010. - DOI
LinkOut - more resources
Full Text Sources

