Discovery of an orally active nitrothiophene-based antitrypanosomal agent
- PMID: 37984297
- PMCID: PMC10843616
- DOI: 10.1016/j.ejmech.2023.115954
Discovery of an orally active nitrothiophene-based antitrypanosomal agent
Abstract
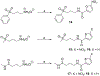
Human African Trypanosomiasis (HAT), caused by Trypanosoma brucei gambiense and rhodesiense, is a parasitic disease endemic to sub-Saharan Africa. Untreated cases of HAT can be severely debilitating and fatal. Although the number of reported cases has decreased progressively over the last decade, the number of effective and easily administered medications is very limited. In this work, we report the antitrypanosomal activity of a series of potent compounds. A subset of molecules in the series are highly selective for trypanosomes and are metabolically stable. One of the compounds, (E)-N-(4-(methylamino)-4-oxobut-2-en-1-yl)-5-nitrothiophene-2-carboxamide (10), selectively inhibited the growth of T. b. brucei, T. b. gambiense and T. b. rhodesiense, have excellent oral bioavailability and was effective in treating acute infection of HAT in mouse models. Based on its excellent bioavailability, compound 10 and its analogs are candidates for lead optimization and pre-clinical investigations.
Keywords: Cysteine protease; HAT; Inhibitors; Trypanosomes; Vinyl sulfone.
Copyright © 2023 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ifedayo Victor Ogungbe reports financial support was provided by National Institute of Health. Ifedayo Victor Ogungbe reports financial support was provided by National Science Foundation. Ifedayo Victor Ogungbe reports a relationship with Biomolecular Science LLC that includes: equity or stocks. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




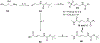
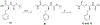
References
-
- Kennedy P. Update on human African trypanosomiasis (sleeping sickness). Journal of Neurology, 2019, 266, 2334–2337. - PubMed
-
- Lindner A; Lejon V; Chappuis F; Seixas J; Kazumba L; Barrett M; Mwamba E; Erphas O; Akl E; Villanueva G; Bergman H; Simarro P; Kadima Ebeja A; Priotto G; Franco J New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: substantial changes for clinical practice. The Lancet Infectious Diseases, 2020, 20, e38–e46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

