Benefit of cardiac resynchronization therapy among older patients: A patient-level meta-analysis
- PMID: 37984672
- PMCID: PMC10842211
- DOI: 10.1016/j.ahj.2023.11.002
Benefit of cardiac resynchronization therapy among older patients: A patient-level meta-analysis
Abstract
Background: Cardiac resynchronization therapy (CRT) reduces heart failure hospitalizations (HFH) and mortality for guideline-indicated patients with heart failure (HF). Most patients with HF are aged ≥70 years but such patients are often under-represented in randomized trials.
Methods: Patient-level data were combined from 8 randomized trials published 2002-2013 comparing CRT to no CRT (n = 6,369). The effect of CRT was estimated using an adjusted Bayesian survival model. Using age as a categorical (<70 vs ≥70 years) or continuous variable, the interaction between age and CRT on the composite end point of HFH or all-cause mortality or all-cause mortality alone was assessed.
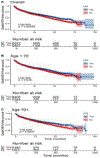
Results: The median age was 67 years with 2436 (38%) being 70+; 1,554 (24%) were women; 2,586 (41%) had nonischemic cardiomyopathy and median QRS duration was 160 ms. Overall, CRT was associated with a delay in time to the composite end point (adjusted hazard ratio [aHR] 0.75, 95% credible interval [CI] 0.66-0.85, P = .002) and all-cause mortality alone (aHR of 0.80, 95% CI 0.69-0.96, P = .017). When age was treated as a categorical variable, there was no interaction between age and the effect of CRT for either end point (P > .1). When age was treated as a continuous variable, older patients appeared to obtain greater benefit with CRT for the composite end point (P for interaction = .027) with a similar but nonsignificant trend for mortality (P for interaction = .35).
Conclusion: Reductions in HFH and mortality with CRT are as great or greater in appropriately indicated older patients. Age should not be a limiting factor for the provision of CRT.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Potential Conflicts of Interest
Dr Zeitler has received research support from the National Institutes of Health, Biosense Webster and Sanofi; consulting fees from Element Sciencies, Abbott, Biosense Webster, and Medtronic; travel from Abbott, Biosense Webster, and Medtronic. Dr Cleland reports grants and personal fees from Pharmacosmos; personal honoraria from Abbott, Astra Zeneca, Idorsia, Myokardia, NI Medical, Novartis, Servier, and Torrent pharmaceuticals; grants and personal honoraria from Amgen/Cytokinetics, Bayer, Bristol Myers Squibb, Johnson & Johnson, Medtronic, Vifor, and Viscardia; personal honoraria and nonfinancial support from Boehringer-Ingelheim outside the submitted work. Dr Curtis serves on medical advisory boards for Janssen Pharmaceuticals, Medtronic, Inc, Abbott, Milestone Pharmaceuticals, and Eagle Pharmaceuticals; and has received honoraria for speaking from Abbott and Medtronic. Dr Friedman has received research support from American Heart Association, Boston Scientific, Biosense Webster, Merit Medical, Medtronic, the National Institutes of Health, and Abbott; and consulting fees from Abbott, AtriCure, Microport, NI Medical, and Sanofi. Dr Gold serves on a medical advisory board for Medtronic and EBR Systems; receives research support to his institution from Boston Scientific, Abbott, and Medtronic; and is a consultant to Boston Scientific and Medtronic. Dr Linde has received research support to her institution from Swedish Heart–Lung Foundation, Swedish Royal Academy of Science, Roche Diagnostics, Astra Zeneca, and Stockholm County Council; and speaker honoraria from Medtronic, Impulse Dynamics, Bayer, Boeringer Ingelheim, Novartis, Vifor Pharma, and Microport. Dr Al-Khatib receives research funding from Medtronic and Boston Scientific through grants to her institution. The other authors report no disclosures.
Figures





References
-
- Dickstein K, Normand C, Auricchio A, Bogale N, Cleland JG, Gitt AK, Stellbrink C, Anker SD, Filippatos G, Gasparini M, et al. CRT Survey II: a European Society of Cardiology survey of cardiac resynchronisation therapy in 11 088 patients-who is doing what to whom and how? European journal of heart failure. 2018;20:1039–1051. doi: 10.1002/ejhf.1142 - DOI - PubMed
-
- Althouse AD, Jain SK, Shalaby A, Singh M, Weiss R, Myaskovsky L, Al-Khatib SM, Saba S. Feasibility of a Randomized Clinical Trial of Cardiac Resynchronization Therapy With or Without an Implantable Defibrillator in Older Patients. Circ Arrhythm Electrophysiol. 2022;15:e010795. doi: 10.1161/CIRCEP.121.010795 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

