Nanotechnology and Cancer Bioelectricity: Bridging the Gap Between Biology and Translational Medicine
- PMID: 37984883
- PMCID: PMC10767462
- DOI: 10.1002/advs.202304110
Nanotechnology and Cancer Bioelectricity: Bridging the Gap Between Biology and Translational Medicine
Abstract
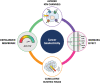
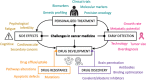
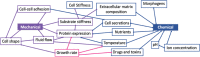
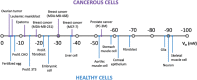
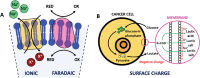
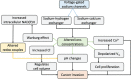
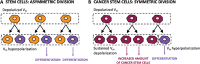
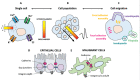
Bioelectricity is the electrical activity that occurs within living cells and tissues. This activity is critical for regulating homeostatic cellular function and communication, and disruptions of the same can lead to a variety of conditions, including cancer. Cancer cells are known to exhibit abnormal electrical properties compared to their healthy counterparts, and this has driven researchers to investigate the potential of harnessing bioelectricity as a tool in cancer diagnosis, prognosis, and treatment. In parallel, bioelectricity represents one of the means to gain fundamental insights on how electrical signals and charges play a role in cancer insurgence, growth, and progression. This review provides a comprehensive analysis of the literature in this field, addressing the fundamentals of bioelectricity in single cancer cells, cancer cell cohorts, and cancerous tissues. The emerging role of bioelectricity in cancer proliferation and metastasis is introduced. Based on the acknowledgement that this biological information is still hard to access due to the existing gap between biological findings and translational medicine, the latest advancements in the field of nanotechnologies for cellular electrophysiology are examined, as well as the most recent developments in micro- and nano-devices for cancer diagnostics and therapy targeting bioelectricity.
Keywords: Bioelectricity; Cancer; Electrophysiology; Ion Channels; Nanotechnology; Non-Excitable Cells; Translational Medicine.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Bioelectricity in dental medicine: a narrative review.Biomed Eng Online. 2024 Jan 3;23(1):3. doi: 10.1186/s12938-023-01189-6. Biomed Eng Online. 2024. PMID: 38172866 Free PMC article. Review.
-
Nanotechnology: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(19):1-43. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074489 Free PMC article.
-
Bioelectricity, Its Fundamentals, Characterization Methodology, and Applications in Nano-Bioprobing and Cancer Diagnosis.Adv Biosyst. 2019 Oct;3(10):e1900101. doi: 10.1002/adbi.201900101. Epub 2019 Aug 27. Adv Biosyst. 2019. PMID: 32648718 Review.
-
Evolution of Bioelectric Membrane Potentials: Implications in Cancer Pathogenesis and Therapeutic Strategies.J Membr Biol. 2024 Dec;257(5-6):281-305. doi: 10.1007/s00232-024-00323-2. Epub 2024 Aug 25. J Membr Biol. 2024. PMID: 39183198 Review.
-
Bioelectricity in Developmental Patterning and Size Control: Evidence and Genetically Encoded Tools in the Zebrafish Model.Cells. 2023 Apr 13;12(8):1148. doi: 10.3390/cells12081148. Cells. 2023. PMID: 37190057 Free PMC article. Review.
Cited by
-
Little strokes fell big oaks: The use of weak magnetic fields and reactive oxygen species to fight cancer.Redox Biol. 2025 Feb;79:103483. doi: 10.1016/j.redox.2024.103483. Epub 2024 Dec 24. Redox Biol. 2025. PMID: 39729909 Free PMC article. Review.
-
Emerging cancer therapies: targeting physiological networks and cellular bioelectrical differences with non-thermal systemic electromagnetic fields in the human body - a comprehensive review.Front Netw Physiol. 2024 Dec 10;4:1483401. doi: 10.3389/fnetp.2024.1483401. eCollection 2024. Front Netw Physiol. 2024. PMID: 39720338 Free PMC article. Review.
-
Recent advances in the halogenated spirooxindoles as novel anticancer scaffolds: chemistry and bioactivity approach.RSC Adv. 2025 Jul 1;15(28):22336-22375. doi: 10.1039/d5ra03404c. eCollection 2025 Jun 30. RSC Adv. 2025. PMID: 40599572 Free PMC article. Review.
-
Deciphering the Regulatory Potential of Antioxidant and Electron-Shuttling Bioactive Compounds in Oolong Tea.Biology (Basel). 2025 Apr 28;14(5):487. doi: 10.3390/biology14050487. Biology (Basel). 2025. PMID: 40427676 Free PMC article.
-
TRP channels and cancer modulation: a voyage beyond metabolic reprogramming, oxidative stress and the advent of nanotechnologies in targeted therapy.J Exp Clin Cancer Res. 2025 Aug 14;44(1):240. doi: 10.1186/s13046-025-03495-4. J Exp Clin Cancer Res. 2025. PMID: 40813985 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
