α-Functionalisation of Cyclic Sulfides Enabled by Lithiation Trapping
- PMID: 37984884
- PMCID: PMC10952194
- DOI: 10.1002/anie.202314423
α-Functionalisation of Cyclic Sulfides Enabled by Lithiation Trapping
Abstract
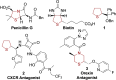
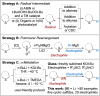
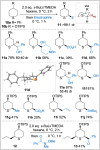
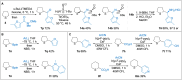
A general and straightforward procedure for the lithiation trapping of cyclic sulfides such as tetrahydrothiophene, tetrahydrothiopyran and a thiomorpholine is described. Trapping with a wide range of electrophiles is demonstrated, leading to more than 50 diverse α-substituted saturated sulfur heterocycles. The methodology provides access to a range of α-substituted cyclic sulfides that are not easily synthesised by the currently available methods.
Keywords: Cyclic Sulfides; Organolithium; α-Functionalization.
© 2023 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

