Synthetic Cells Revisited: Artificial Cells Construction Using Polymeric Building Blocks
- PMID: 37984885
- PMCID: PMC10885666
- DOI: 10.1002/advs.202305837
Synthetic Cells Revisited: Artificial Cells Construction Using Polymeric Building Blocks
Abstract
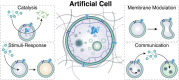
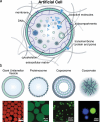
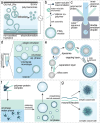
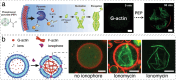
The exponential growth of research on artificial cells and organelles underscores their potential as tools to advance the understanding of fundamental biological processes. The bottom-up construction from a variety of building blocks at the micro- and nanoscale, in combination with biomolecules is key to developing artificial cells. In this review, artificial cells are focused upon based on compartments where polymers are the main constituent of the assembly. Polymers are of particular interest due to their incredible chemical variety and the advantage of tuning the properties and functionality of their assemblies. First, the architectures of micro- and nanoscale polymer assemblies are introduced and then their usage as building blocks is elaborated upon. Different membrane-bound and membrane-less compartments and supramolecular structures and how they combine into advanced synthetic cells are presented. Then, the functional aspects are explored, addressing how artificial organelles in giant compartments mimic cellular processes. Finally, how artificial cells communicate with their surrounding and each other such as to adapt to an ever-changing environment and achieve collective behavior as a steppingstone toward artificial tissues, is taken a look at. Engineering artificial cells with highly controllable and programmable features open new avenues for the development of sophisticated multifunctional systems.
Keywords: artificial cells; artificial organelles; artificial signaling; bottom-up; collective behavior; communicative networks; polymers.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dos Santos E. C., Angelini A., Hürlimann D., Meier W., Palivan C. G., Chemistry 2020, 2, 470.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
