Evaluation of Rhodanine Indolinones as AANAT Inhibitors
- PMID: 37984928
- PMCID: PMC10843758
- DOI: 10.1002/cmdc.202300567
Evaluation of Rhodanine Indolinones as AANAT Inhibitors
Abstract
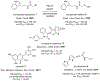
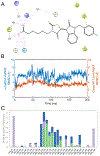
Circadian rhythm (CR) dysregulation negatively impacts health and contributes to mental disorders. The role of melatonin, a hormone intricately linked to CR, is still a subject of active study. The enzyme arylalkylamine N-acetyltransferase (AANAT) is responsible for melatonin synthesis, and it is a potential target for disorders that involve abnormally high melatonin levels, such as seasonal affective disorder (SAD). Current AANAT inhibitors suffer from poor cell permeability, selectivity, and/or potency. To address the latter, we have employed an X-ray crystal-based model to guide the modification of a previously described AANAT inhibitor, containing a rhodanine-indolinone core. We made various structural modifications to the core structure, including testing the importance of a carboxylic acid group thought to bind in the CoA site, and we evaluated these changes using MD simulations in conjunction with enzymatic assay data. Additionally, we tested three AANAT inhibitors in a zebrafish locomotion model to determine their effects in vivo. Key discoveries were that potency could be modestly improved by replacing a 5-carbon alkyl chain with rings and that the central rhodanine ring could be replaced by other heterocycles and maintain potency.
Keywords: acetyl transferase; circadian rhythm; enzyme models; heterocycles; melatonin.
© 2023 Wiley-VCH GmbH.
Figures
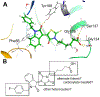
 hydrogen bonds;
hydrogen bonds;  hydrophobic;
hydrophobic;  ionic;
ionic;  water bridges.
water bridges.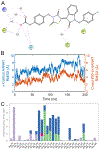
 hydrogen bonds;
hydrogen bonds;  hydrophobic;
hydrophobic;  ionic;
ionic;  water bridges.
water bridges.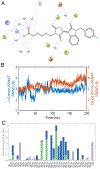
 hydrogen bonds;
hydrogen bonds;  hydrophobic;
hydrophobic;  ionic;
ionic;  water bridges.
water bridges.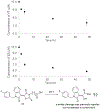
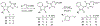
Similar articles
-
De novo discovery of serotonin N-acetyltransferase inhibitors.J Med Chem. 2007 Nov 1;50(22):5330-8. doi: 10.1021/jm0706463. Epub 2007 Oct 9. J Med Chem. 2007. PMID: 17924613 Free PMC article.
-
The arylalkylamine-N-acetyltransferase (AANAT) acetylates dopamine in the digestive tract of goldfish: a role in intestinal motility.Neurochem Int. 2013 May;62(6):873-80. doi: 10.1016/j.neuint.2013.02.023. Epub 2013 Mar 4. Neurochem Int. 2013. PMID: 23466408
-
Heterogeneous ribonucleoprotein R regulates arylalkylamine N-acetyltransferase synthesis via internal ribosomal entry site-mediated translation in a circadian manner.J Pineal Res. 2015 Nov;59(4):518-29. doi: 10.1111/jpi.12284. Epub 2015 Oct 25. J Pineal Res. 2015. PMID: 26444903
-
[14-3-3 proteins--a role in the regulation of melatonin biosynthesis].Postepy Biochem. 2006;52(1):35-41. Postepy Biochem. 2006. PMID: 16869299 Review. Polish.
-
Circadian and Neuroendocrine Basis of Photoperiodism Controlling Diapause in Insects and Mites: A Review.Front Physiol. 2022 Jun 22;13:867621. doi: 10.3389/fphys.2022.867621. eCollection 2022. Front Physiol. 2022. PMID: 35812309 Free PMC article. Review.
Cited by
-
Backbone resonance assignments of dopamine N-acetyltransferase in free and cofactor-bound states.Biomol NMR Assign. 2025 Jun;19(1):83-93. doi: 10.1007/s12104-025-10222-9. Epub 2025 Feb 12. Biomol NMR Assign. 2025. PMID: 39934620 Free PMC article.
-
AANAT kinetics of CoASH-targeted electrophiles of tryptamine and related analogs.Bioorg Med Chem Lett. 2024 Nov 15;113:129975. doi: 10.1016/j.bmcl.2024.129975. Epub 2024 Sep 25. Bioorg Med Chem Lett. 2024. PMID: 39332648
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

