Single-facility study of the effectiveness of rehabilitation therapy using wearable hybrid assistive limb for patients with bleeding disorders: study protocol for a randomised controlled trial
- PMID: 37984952
- PMCID: PMC10660193
- DOI: 10.1136/bmjopen-2023-076153
Single-facility study of the effectiveness of rehabilitation therapy using wearable hybrid assistive limb for patients with bleeding disorders: study protocol for a randomised controlled trial
Abstract
Introduction: Haemophilic arthropathy, a serious complication of haemophilia, results from recurrent joint bleeding, causing progressive joint damage and severely impacting patient quality of life. Rehabilitation therapy (RT) effectively addresses declining physical function due to joint degradation, but pain during RT can hinder its success. Therefore, an effective pain-alleviating treatment method is required. The single-joint hybrid assistive limb (HAL-SJ), a powered exoskeleton, measures bioelectric potential during muscle contraction and provides motorised support, potentially alleviating pain.
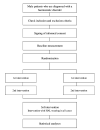
Objective: This study outlines our protocol for a randomised, prospective, single-blind (evaluator) trial aimed to investigate the effects of HAL-SJ on pain reduction during RT, kinesiophobia and other physical functions in patients with haemophilia.
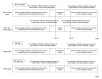
Methods and analysis: This two-group comparison intervention study will include 24 male patients aged 12-85 years diagnosed with a bleeding disorder necessitating RT for pain and physical function improvement. The primary outcome measures pain changes during the first and second RT session in patients receiving HAL-SJ-assisted RT compared with traditional RT without HAL-SJ. The secondary outcomes include kinesiophobia (Japanese version of the Tampa Scale for Kinesiophobia), standing position gait (zebris FDM-T treadmill), range of motion (manual goniometer) and body surface temperature (infrared thermography camera) during the study period of up to 3 months or until the end of 10 RTs. RT intensity remains below that required to move the affected joint against gravity, given HAL-SJ's muscular support. The follow-up period extends to 1 month after the last RT. Intergroup study variables are compared by an unpaired t-test or Mann-Whitney test. Intragroup comparisons of secondary outcomes are analysed by a paired t-test or Wilcoxon signed-rank test.
Ethics and dissemination: This study was approved by the accreditation committee of Nara Medical University Hospital. The study results will disseminate through publication in a peer-reviewed journal.
Trial registration number: jRCTs052220076.
Keywords: Haemophilia, hybrid assistive limb; bleeding disorders; haemophilic arthropathy; rehabilitation therapy; single-joint limb.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: YM, AS, KT and MS: members of Medicinal Biology of Thrombosis and Hemostasis established by Nara Medical University and Chugai Pharmaceutical Co., Ltd. MS: patents for inventions relating to products of Chugai Pharmaceutical Co., Ltd. YI: grants or research support from Chugai Pharmaceutical Co., Ltd. and Olympus Co., Ltd. DS and AK: no conflict of interest. KT: grants or research support from Japan Blood Products Organisation, The Mother and Child Health Foundation and Novo Nordisk Pharma. KN: grants or research support from Chugai Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; KM Biologics Co., Ltd.; Sanofi Co., Ltd; Novo Nordisk Pharma Co., Ltd; Bayer Co., Ltd.; AbbVie GK LLC; and Janssen Pharmaceutical K.K. Co., Ltd; honoraria or consultation fees from Chugai Pharmaceutical Co., Ltd.; Sanofi Co., Ltd; and CSL Behring. YT: grants from Chugai Pharmaceutical Co., Ltd. and Asahi Kasei Co., Ltd. MS: representative of Medicinal Biology of Thrombosis and Hemostasis collaborative research laboratory; research support from Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd. and CSL Behring; honoraria or consultation fees from Chugai Pharmaceutical Co., Ltd.; speaker’s bureau from Chugai Pharmaceutical Co., Ltd.; CSL Behring; Sanofi; Bayer; Novo Nordisk Pharma; Takeda Pharmaceutical Co., Ltd.; Pfizer; and Fujimoto Seiyaku Corp.
Figures

References
-
- Deni̇z V, Atalay Güzel N. Do therapeutic exercises improve kinesophobia and health-related quality of life in adult hemophilia patients? A randomised controlled trial. Int J Disabil Sports Health Sci 2020;3:11–9. 10.33438/ijdshs.690280 - DOI
-
- Aykar S, Can F, Sahin F. Relationship between fear of movement and physical activity levels in adult hemophilic individuals. Ann Med Res 2020;27:219. 10.5455/annalsmedres.2019.10.603 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
