Mixed Selectivity Coding of Content-Temporal Detail by Dorsomedial Posterior Parietal Neurons
- PMID: 37985178
- PMCID: PMC10860630
- DOI: 10.1523/JNEUROSCI.1677-23.2023
Mixed Selectivity Coding of Content-Temporal Detail by Dorsomedial Posterior Parietal Neurons
Abstract
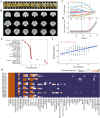
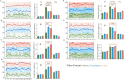
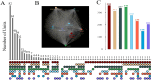
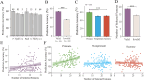
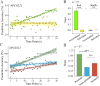
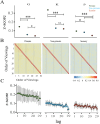
The dorsomedial posterior parietal cortex (dmPPC) is part of a higher-cognition network implicated in elaborate processes underpinning memory formation, recollection, episode reconstruction, and temporal information processing. Neural coding for complex episodic processing is however under-documented. Here, we recorded extracellular neural activities from three male rhesus macaques (Macaca mulatta) and revealed a set of neural codes of "neuroethogram" in the primate parietal cortex. Analyzing neural responses in macaque dmPPC to naturalistic videos, we discovered several groups of neurons that are sensitive to different categories of ethogram items, low-level sensory features, and saccadic eye movement. We also discovered that the processing of category and feature information by these neurons is sustained by the accumulation of temporal information over a long timescale of up to 30 s, corroborating its reported long temporal receptive windows. We performed an additional behavioral experiment with additional two male rhesus macaques and found that saccade-related activities could not account for the mixed neuronal responses elicited by the video stimuli. We further observed monkeys' scan paths and gaze consistency are modulated by video content. Taken altogether, these neural findings explain how dmPPC weaves fabrics of ongoing experiences together in real time. The high dimensionality of neural representations should motivate us to shift the focus of attention from pure selectivity neurons to mixed selectivity neurons, especially in increasingly complex naturalistic task designs.
Keywords: dorsomedial posterior parietal cortex; information accumulation; mixed selective representation; neuroethology; scan path and gaze consistency; temporal receptive window.
Copyright © 2024 the authors.
Figures




References
-
- Adams G (2014) Tinbergen alpha. Release 1. 10.5281/zenodo.13009. http://zenodo.org/record/13009#.VNj3Ji7w-7A - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
