From Liquid to Solid-State Lithium Metal Batteries: Fundamental Issues and Recent Developments
- PMID: 37985522
- PMCID: PMC10661211
- DOI: 10.1007/s40820-023-01234-y
From Liquid to Solid-State Lithium Metal Batteries: Fundamental Issues and Recent Developments
Abstract
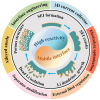
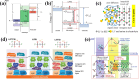
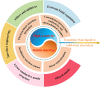
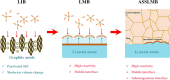
The widespread adoption of lithium-ion batteries has been driven by the proliferation of portable electronic devices and electric vehicles, which have increasingly stringent energy density requirements. Lithium metal batteries (LMBs), with their ultralow reduction potential and high theoretical capacity, are widely regarded as the most promising technical pathway for achieving high energy density batteries. In this review, we provide a comprehensive overview of fundamental issues related to high reactivity and migrated interfaces in LMBs. Furthermore, we propose improved strategies involving interface engineering, 3D current collector design, electrolyte optimization, separator modification, application of alloyed anodes, and external field regulation to address these challenges. The utilization of solid-state electrolytes can significantly enhance the safety of LMBs and represents the only viable approach for advancing them. This review also encompasses the variation in fundamental issues and design strategies for the transition from liquid to solid electrolytes. Particularly noteworthy is that the introduction of SSEs will exacerbate differences in electrochemical and mechanical properties at the interface, leading to increased interface inhomogeneity-a critical factor contributing to failure in all-solid-state lithium metal batteries. Based on recent research works, this perspective highlights the current status of research on developing high-performance LMBs.
Keywords: All-solid-state lithium metal battery; Interface; Li dendrite; Lithium metal batteries; Solid electrolyte.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no interest conflict. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures















References
-
- Y. Nishi, Lithium ion secondary batteries; past 10 years and the future. J. Power. Sources 100(1), 101–106 (2001). 10.1016/S0378-7753(01)00887-4
-
- B. Dunn, H. Kamath, J.-M. Tarascon, Electrical energy storage for the grid: a battery of choices. Science 334(6058), 928–935 (2011). 10.1126/science.1212741 - PubMed
-
- J.M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries. Nature 414(6861), 359–367 (2001). 10.1038/35104644 - PubMed
-
- Y. Huang, J. Li, Key challenges for grid-scale lithium-ion battery energy storage. Adv. Energy Mater. 12(48), 2202197 (2022). 10.1002/aenm.202202197
-
- T. Waldmann, R.-G. Scurtu, K. Richter, M. Wohlfahrt-Mehrens, 18650 vs. 21700 Li-ion cells–a direct comparison of electrochemical, thermal, and geometrical properties. J. Power. Sources 472, 228614 (2020). 10.1016/j.jpowsour.2020.228614
Publication types
LinkOut - more resources
Full Text Sources
