STAG2 Regulates Homologous Recombination Repair and Sensitivity to ATM Inhibition
- PMID: 37985839
- PMCID: PMC10754142
- DOI: 10.1002/advs.202302494
STAG2 Regulates Homologous Recombination Repair and Sensitivity to ATM Inhibition
Abstract
Stromal antigen 2 (STAG2), a subunit of the cohesin complex, is recurrently mutated in various tumors. However, the role of STAG2 in DNA repair and its therapeutic implications are largely unknown. Here it is reported that knockout of STAG2 results in increased double-stranded breaks (DSBs) and chromosomal aberrations by reducing homologous recombination (HR) repair, and confers hypersensitivity to inhibitors of ataxia telangiectasia mutated (ATMi), Poly ADP Ribose Polymerase (PARPi), or the combination of both. Of note, the impaired HR by STAG2-deficiency is mainly attributed to the restored expression of KMT5A, which in turn methylates H4K20 (H4K20me0) to H4K20me1 and thereby decreases the recruitment of BRCA1-BARD1 to chromatin. Importantly, STAG2 expression correlates with poor prognosis of cancer patients. STAG2 is identified as an important regulator of HR and a potential therapeutic strategy for STAG2-mutant tumors is elucidated.
Keywords: ATM inhibitors; PARP inhibitors; STAG2; homologous recombination; synthetic lethality.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
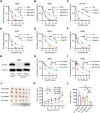

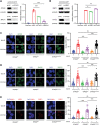



References
Publication types
MeSH terms
Substances
Grants and funding
- 81972227/National Natural Science Foundation of China
- 82172646/National Natural Science Foundation of China
- 81872001/National Natural Science Foundation of China
- 82073189/National Natural Science Foundation of China
- 82202905/National Natural Science Foundation of China
- 82373190/National Natural Science Foundation of China
- 2020B1212060023/Science and Technology Planning Project of Guangdong Province
- M202108/Guangdong Esophageal Cancer Institute Science and Technology Program
- SL2022A04J01968/2023 Guangzhou Basic and Applied Basic Research Project
- 2021YFA1300201/National Key R&D Program of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
