Environmentally friendly catalyst- and solvent-free synthesis of 2-anilino nicotinic acids derivatives as potential lead COX inhibitors
- PMID: 37986120
- PMCID: PMC10662667
- DOI: 10.1186/s13065-023-01078-y
Environmentally friendly catalyst- and solvent-free synthesis of 2-anilino nicotinic acids derivatives as potential lead COX inhibitors
Abstract
In this study, an environmentally friendly, solvent- and catalyst-free synthesis of 2-anilino nicotinic acids derivatives is reported. This operationally simple and green procedure was applied to a selection of primary aromatic amines giving rise to 23 derivatives of 2-anilino nicotinic acids in a very short reaction time (15-120 min) with good to excellent yield. Next, similarity searches were executed on these derivatives to find the possible biological target. These products were screened for inhibition of COX-1 and COX-2 by molecular docking and dynamic studies. In silico studies revealed that among these derivatives, the structure 10 bearing meta-chlorine substitutions could act as COX-1 and COX-2 inhibitors. These results can be used in designing important lead compounds for further development as potential anti-inflammatory drugs.
Keywords: 2-Anilino nicotinic acids; COX; Molecular dynamic simulations; Similarity search; Solvent -free synthesis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
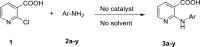

References
-
- Osigbemhe IG, Louis H, Khan EM, Etim EE, Oyo-Ita EE, Oviawe AP, Edet HO, Obuye F. Antibacterial potential of 2-(-(2-Hydroxyphenyl)-methylidene)-amino) nicotinic acid: experimental, DFT studies, and molecular docking approach. Appl Biochem Biotechnol. 2022;194(12):5680–5701. doi: 10.1007/s12010-022-04054-9. - DOI - PubMed
-
- Li Z, Xiao S, Liang R, Xia Z. Synthesis of 2-(arylamino) nicotinic acids in high-temperature water. Res Chem Intermed. 2012;38:1691–1697. doi: 10.1007/s11164-012-0494-0. - DOI
-
- Iwasaki N, Ohashi T, Musoh K, Nishino H, Kado N, Yasuda S, Kato H, Ito Y. Amphoteric drugs 3-[(5, 11-dihydro [1] benzoxepino [4, 3-b] pyridin-11-ylidene) piperidino] Synthesis and antiallergic activity of propionic acid derivatives and related compounds. J Med Chem. 1995;38(3):496–507. doi: 10.1021/jm00003a013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
