The O-GlcNAc dichotomy: when does adaptation become pathological?
- PMID: 37986614
- PMCID: PMC12083504
- DOI: 10.1042/CS20220309
The O-GlcNAc dichotomy: when does adaptation become pathological?
Abstract
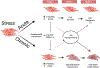
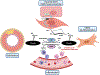
O-Linked attachment of β-N-acetylglucosamine (O-GlcNAc) on serine and threonine residues of nuclear, cytoplasmic, and mitochondrial proteins is a highly dynamic and ubiquitous post-translational modification that impacts the function, activity, subcellular localization, and stability of target proteins. Physiologically, acute O-GlcNAcylation serves primarily to modulate cellular signaling and transcription regulatory pathways in response to nutrients and stress. To date, thousands of proteins have been revealed to be O-GlcNAcylated and this number continues to grow as the technology for the detection of O-GlcNAc improves. The attachment of a single O-GlcNAc is catalyzed by the enzyme O-GlcNAc transferase (OGT), and their removal is catalyzed by O-GlcNAcase (OGA). O-GlcNAcylation is regulated by the metabolism of glucose via the hexosamine biosynthesis pathway, and the metabolic abnormalities associated with pathophysiological conditions are all associated with increased flux through this pathway and elevate O-GlcNAc levels. While chronic O-GlcNAcylation is well associated with cardiovascular dysfunction, only until recently, and with genetically modified animals, has O-GlcNAcylation as a contributing mechanism of cardiovascular disease emerged. This review will address and critically evaluate the current literature on the role of O-GlcNAcylation in vascular physiology, with a view that this pathway can offer novel targets for the treatment and prevention of cardiovascular diseases.
Keywords: O-GlcNAc; cardiovascular physiology; intracellular signaling.
© 2023 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

