Hallmarks of ageing in human skeletal muscle and implications for understanding the pathophysiology of sarcopenia in women and men
- PMID: 37986616
- PMCID: PMC10665130
- DOI: 10.1042/CS20230319
Hallmarks of ageing in human skeletal muscle and implications for understanding the pathophysiology of sarcopenia in women and men
Abstract
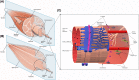
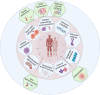
Ageing is a complex biological process associated with increased morbidity and mortality. Nine classic, interdependent hallmarks of ageing have been proposed involving genetic and biochemical pathways that collectively influence ageing trajectories and susceptibility to pathology in humans. Ageing skeletal muscle undergoes profound morphological and physiological changes associated with loss of strength, mass, and function, a condition known as sarcopenia. The aetiology of sarcopenia is complex and whilst research in this area is growing rapidly, there is a relative paucity of human studies, particularly in older women. Here, we evaluate how the nine classic hallmarks of ageing: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication contribute to skeletal muscle ageing and the pathophysiology of sarcopenia. We also highlight five novel hallmarks of particular significance to skeletal muscle ageing: inflammation, neural dysfunction, extracellular matrix dysfunction, reduced vascular perfusion, and ionic dyshomeostasis, and discuss how the classic and novel hallmarks are interconnected. Their clinical relevance and translational potential are also considered.
Keywords: hallmarks of ageing; sarcopenia; skeletal muscle.
© 2023 The Author(s).
Conflict of interest statement
A.G., K.S., M.G., and A.A.S. declare no competing interests associated with the manuscript. T.S. is an employee of Regeneron and holds stock in Regeneron.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

