The MT1 receptor as the target of ramelteon neuroprotection in ischemic stroke
- PMID: 37986632
- PMCID: PMC10872556
- DOI: 10.1111/jpi.12925
The MT1 receptor as the target of ramelteon neuroprotection in ischemic stroke
Abstract
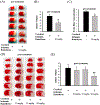
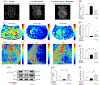
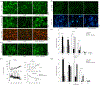
Stroke is the leading cause of death and disability worldwide. Novel and effective therapies for ischemic stroke are urgently needed. Here, we report that melatonin receptor 1A (MT1) agonist ramelteon is a neuroprotective drug candidate as demonstrated by comprehensive experimental models of ischemic stroke, including a middle cerebral artery occlusion (MCAO) mouse model of cerebral ischemia in vivo, organotypic hippocampal slice cultures ex vivo, and cultured neurons in vitro; the neuroprotective effects of ramelteon are diminished in MT1-knockout (KO) mice and MT1-KO cultured neurons. For the first time, we report that the MT1 receptor is significantly depleted in the brain of MCAO mice, and ramelteon treatment significantly recovers the brain MT1 losses in MCAO mice, which is further explained by the Connectivity Map L1000 bioinformatic analysis that shows gene-expression signatures of MCAO mice are negatively connected to melatonin receptor agonist like Ramelteon. We demonstrate that ramelteon improves the cerebral blood flow signals in ischemic stroke that is potentially mediated, at least, partly by mechanisms of activating endothelial nitric oxide synthase. Our results also show that the neuroprotection of ramelteon counteracts reactive oxygen species-induced oxidative stress and activates the nuclear factor erythroid 2-related factor 2/heme oxygenase-1 pathway. Ramelteon inhibits the mitochondrial and autophagic death pathways in MCAO mice and cultured neurons, consistent with gene set enrichment analysis from a bioinformatics perspective angle. Our data suggest that Ramelteon is a potential neuroprotective drug candidate, and MT1 is the neuroprotective target for ischemic stroke, which provides new insights into stroke therapy. MT1-KO mice and cultured neurons may provide animal and cellular models of accelerated ischemic damage and neuronal cell death.
Keywords: CBF; MRI; MT1 receptor; MT1−/− cultured neurons; MT1−/− mice; Nrf2/HO-1; ROS; Ramelteon; bioinformatics; ischemic stroke; mitochondrial and autophagic death pathways; p-eNOS/eNOS.
© 2023 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures





References
-
- Zhou H, Wang J, Jiang J, Stavrovskaya IG, Li M, Li W, Wu Q, Zhang X, Luo C, Zhou S, Sirianni AC, Sarkar S, Kristal BS, Friedlander RM, Wang X. N-acetyl-serotonin offers neuroprotection through inhibiting mitochondrial death pathways and autophagic activation in experimental models of ischemic injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34 - PMC - PubMed
-
- Sinha B, Wu Q, Li W, Tu Y, Sirianni AC, Chen Y, Jiang J, Zhang X, Chen W, Zhou S, Reiter RJ, Manning SM, Patel NJ, Aziz-Sultan AM, Inder TE, Friedlander RM, Fu J, Wang X. Protection of melatonin in experimental models of newborn hypoxic-ischemic brain injury through mt1 receptor. Journal of pineal research. 2018;64 - PubMed
-
- Zhang Y, Cook A, Kim J, Baranov SV, Jiang J, Smith K, Cormier K, Bennett E, Browser RP, Day AL, Carlisle DL, Ferrante RJ, Wang X, Friedlander RM. Melatonin inhibits the caspase-1/cytochrome c/caspase-3 cell death pathway, inhibits mt1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2013;55:26–35 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

