This is a preprint.
Concurrent RB1 loss and BRCA-deficiency predicts enhanced immunological response and long-term survival in tubo-ovarian high-grade serous carcinoma
- PMID: 37986741
- PMCID: PMC10659507
- DOI: 10.1101/2023.11.09.23298321
Concurrent RB1 loss and BRCA-deficiency predicts enhanced immunological response and long-term survival in tubo-ovarian high-grade serous carcinoma
Update in
-
Concurrent RB1 Loss and BRCA Deficiency Predicts Enhanced Immunologic Response and Long-term Survival in Tubo-ovarian High-grade Serous Carcinoma.Clin Cancer Res. 2024 Aug 15;30(16):3481-3498. doi: 10.1158/1078-0432.CCR-23-3552. Clin Cancer Res. 2024. PMID: 38837893 Free PMC article.
Abstract
Background: Somatic loss of the tumour suppressor RB1 is a common event in tubo-ovarian high-grade serous carcinoma (HGSC), which frequently co-occurs with alterations in homologous recombination DNA repair genes including BRCA1 and BRCA2 (BRCA). We examined whether tumour expression of RB1 was associated with survival across ovarian cancer histotypes (HGSC, endometrioid (ENOC), clear cell (CCOC), mucinous (MOC), low-grade serous carcinoma (LGSC)), and how co-occurrence of germline BRCA pathogenic variants and RB1 loss influences long-term survival in a large series of HGSC.
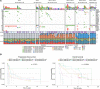
Patients and methods: RB1 protein expression patterns were classified by immunohistochemistry in epithelial ovarian carcinomas of 7436 patients from 20 studies participating in the Ovarian Tumor Tissue Analysis consortium and assessed for associations with overall survival (OS), accounting for patient age at diagnosis and FIGO stage. We examined RB1 expression and germline BRCA status in a subset of 1134 HGSC, and related genotype to survival, tumour infiltrating CD8+ lymphocyte counts and transcriptomic subtypes. Using CRISPR-Cas9, we deleted RB1 in HGSC cell lines with and without BRCA1 mutations to model co-loss with treatment response. We also performed genomic analyses on 126 primary HGSC to explore the molecular characteristics of concurrent homologous recombination deficiency and RB1 loss.
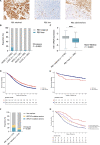
Results: RB1 protein loss was most frequent in HGSC (16.4%) and was highly correlated with RB1 mRNA expression. RB1 loss was associated with longer OS in HGSC (hazard ratio [HR] 0.74, 95% confidence interval [CI] 0.66-0.83, P = 6.8 ×10-7), but with poorer prognosis in ENOC (HR 2.17, 95% CI 1.17-4.03, P = 0.0140). Germline BRCA mutations and RB1 loss co-occurred in HGSC (P < 0.0001). Patients with both RB1 loss and germline BRCA mutations had a superior OS (HR 0.38, 95% CI 0.25-0.58, P = 5.2 ×10-6) compared to patients with either alteration alone, and their median OS was three times longer than non-carriers whose tumours retained RB1 expression (9.3 years vs. 3.1 years). Enhanced sensitivity to cisplatin (P < 0.01) and paclitaxel (P < 0.05) was seen in BRCA1 mutated cell lines with RB1 knockout. Among 126 patients with whole-genome and transcriptome sequence data, combined RB1 loss and genomic evidence of homologous recombination deficiency was correlated with transcriptional markers of enhanced interferon response, cell cycle deregulation, and reduced epithelial-mesenchymal transition in primary HGSC. CD8+ lymphocytes were most prevalent in BRCA-deficient HGSC with co-loss of RB1.
Conclusions: Co-occurrence of RB1 loss and BRCA mutation was associated with exceptionally long survival in patients with HGSC, potentially due to better treatment response and immune stimulation.
Conflict of interest statement
COMPETING INTERESTS DDLB is an Exo Therapeutics advisor and has received research grant funding from AstraZeneca, Genentech-Roche and BeiGene for unrelated work. SF, NT, KA, and ADeF received grant funding from AstraZeneca for unrelated work. AGM and UM report funded research collaborations for unrelated work with industry: Intelligent Lab on Fiber, RNA Guardian, Micronoma and Mercy BioAnalytics. UM had stock ownership (2011–2021) awarded by University College London (UCL) in Abcodia, which held the licence for the Risk of Ovarian Cancer Algorithm (ROCA). UM reports research collaboration contracts with Cambridge University and QIMR Berghofer Medical Research Institute. UM holds patent number EP10178345.4 for Breast Cancer Diagnostics. UM is a member of Tina’s Wish Scientific Advisory Board (USA) and Research Advisory Panel, Yorkshire Cancer Research (UK). The remaining authors declared no conflicts of interest.
Figures


References
-
- Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015; 521(7553): 489–94. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
