This is a preprint.
Post-Vaccination Syndrome: A Descriptive Analysis of Reported Symptoms and Patient Experiences After Covid-19 Immunization
- PMID: 37986769
- PMCID: PMC10659483
- DOI: 10.1101/2023.11.09.23298266
Post-Vaccination Syndrome: A Descriptive Analysis of Reported Symptoms and Patient Experiences After Covid-19 Immunization
Abstract
Introduction: A chronic post-vaccination syndrome (PVS) after covid-19 vaccination has been reported but has yet to be well characterized.
Methods: We included 241 individuals aged 18 and older who self-reported PVS after covid-19 vaccination and who joined the online Yale Listen to Immune, Symptom and Treatment Experiences Now (LISTEN) Study from May 2022 to July 2023. We summarized their demographics, health status, symptoms, treatments tried, and overall experience.
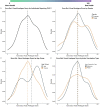
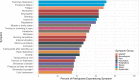
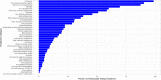
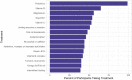
Results: The median age of participants was 46 years (interquartile range [IQR]: 38 to 56), with 192 (80%) identifying as female, 209 (87%) as non-Hispanic White, and 211 (88%) from the United States. Among these participants with PVS, 127 (55%) had received the BNT162b2 [Pfizer-BioNTech] vaccine, and 86 (37%) received the mRNA-1273 [Moderna] vaccine. The median time from the day of index vaccination to symptom onset was three days (IQR: 1 day to 8 days). The time from vaccination to symptom survey completion was 595 days (IQR: 417 to 661 days). The median Euro-QoL visual analogue scale score was 50 (IQR: 39 to 70). The five most common symptoms were exercise intolerance (71%), excessive fatigue (69%), numbness (63%), brain fog (63%), and neuropathy (63%). In the week before survey completion, participants reported feeling unease (93%), fearfulness (82%), and overwhelmed by worries (81%), as well as feelings of helplessness (80%), anxiety (76%), depression (76%), hopelessness (72%), and worthlessness (49%) at least once. Participants reported a median of 20 (IQR: 13 to 30) interventions to treat their condition.
Conclusions: In this study, individuals who reported PVS after covid-19 vaccination had low health status, high symptom burden, and high psychosocial stress despite trying many treatments. There is a need for continued investigation to understand and treat this condition.
Figures
References
-
- Twentyman E, Wallace M, Roper LE, et al. Interim recommendation of the advisory committee on immunization practices for use of the Novavax COVID-19 vaccine in persons aged ≥18 years - United States, July 2022. MMWR Morb Mortal Wkly Rep 2022;71(31):988–92. doi: 10.15585/mmwr.mm7131a2 [published Online First: 20220805] - DOI - PMC - PubMed
-
- Pillay J, Gaudet L, Wingert A, et al. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: Living evidence syntheses and review. BMJ 2022;378:e069445. doi: 10.1136/bmj-2021-069445 [published Online First: 20220713] - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
