This is a preprint.
Divergent reward cue representations in prefrontal cortex underlie differences in reward motivation between adolescents and adults
- PMID: 37986789
- PMCID: PMC10659319
- DOI: 10.1101/2023.11.07.565069
Divergent reward cue representations in prefrontal cortex underlie differences in reward motivation between adolescents and adults
Abstract
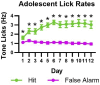
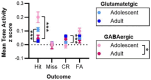
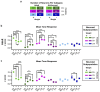
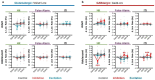
A prevailing view on postnatal brain development is that brain regions gradually acquire adult functions as they mature. The medial prefrontal cortex (mPFC) regulates reward learning, motivation, and behavioral inhibition, and undergoes a protracted postnatal maturation. During adolescence, reward-seeking behavior is heightened compared to adulthood - a developmental difference that may be driven by a hypoactive mPFC, with decreased top-down control of impulsive reward-seeking. However, this hypothesis has been difficult to test directly, due in part to technical challenges of recording neuronal activity in vivo across this developmental period. Here, using a novel 2-photon imaging-compatible platform for recording mPFC activity during an operant reward conditioning task beginning early in life, we show that the adolescent mPFC is hyper-responsive to reward cues. Distinct populations of mPFC neurons encode reward-predictive cues across development, but representations of no-reward cues and unrewarded outcomes are relatively muted in adolescence. Chemogenetic inhibition of GABAergic neurons decreased motivation in adolescence but not in adulthood. Together, our findings indicate that reward-related activity in the adolescent mPFC does not gradually increase across development. On the contrary, adolescent mPFC neurons are hyper-responsive to reward-related stimuli and encode reward-predictive cues and outcomes through qualitatively different mechanisms relative to the adult mPFC, opening avenues to developing distinct, developmentally informed strategies for modulating reward-seeking behavior in adolescence and adulthood.
Keywords: 2-Photon Calcium Imaging; Adolescence; Chemogenetics; Development; Medial prefrontal cortex; Motivation; Neuronal Decoding; Reward Learning.
Figures








References
-
- Whelan R. et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat. Neurosci. 15, 920–925 (2012). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
